Search Results
Results for: 'acidosis'
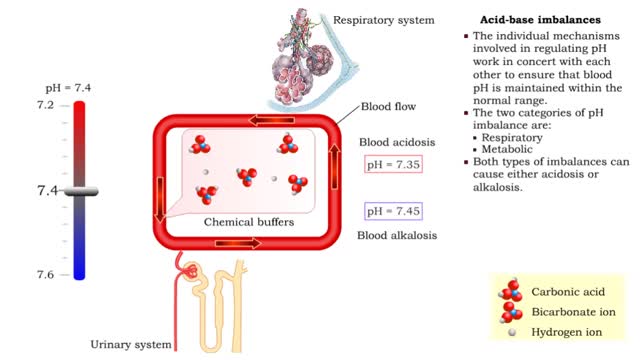
Acid-base imbalances - respiratory acidosis and alkalosis
By: HWC, Views: 12047
• The individual mechanisms involved in regulating pH work in concert with each other to ensure that blood pH is maintained within the normal range. • The two categories of pH imbalance are: • Respiratory • Metabolic • Both types of imbalances can cause either acidosis or alka...
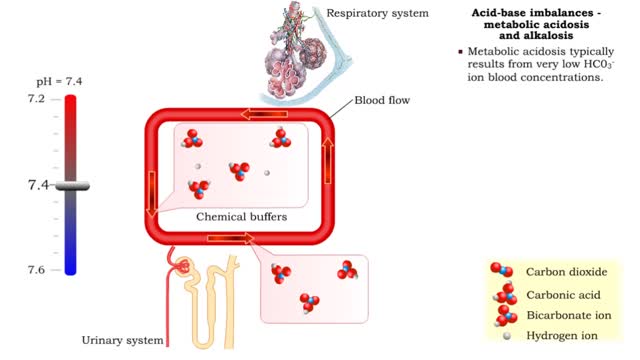
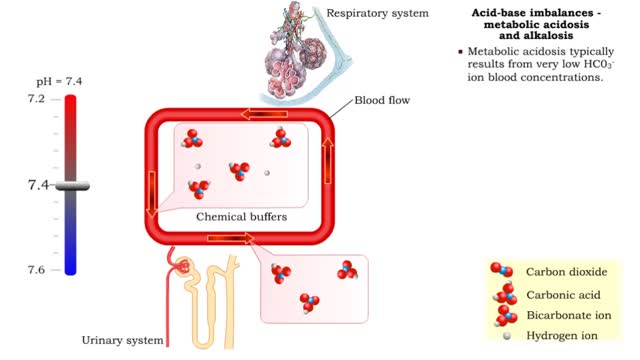
Acid-base imbalances - metabolic acidosis and alkalosis
By: HWC, Views: 11860
• Metabolic acidosis typically results from very low HCO3- ion blood concentrations. • Metabolic alkalosis typically results from very high HCO3- ion blood concentrations.
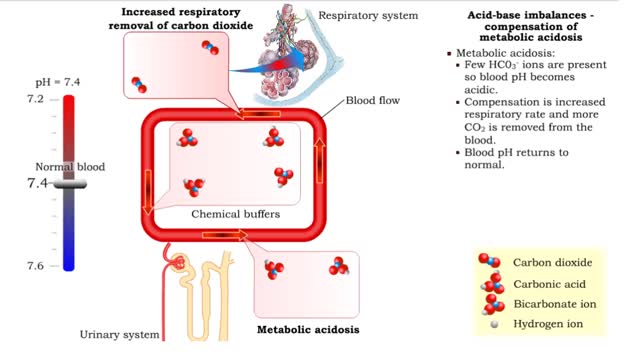
Acid-base imbalances - compensation of metabolic acidosis and alkalosis
By: HWC, Views: 11856
1. Metabolic acidosis: • Few HC03- ions are present so blood pH becomes acidic. • Compensation is increased respiratory rate and more CO2 is removed from the blood. • Blood pH returns to normal. 2. Metabolic alkalosis: • Many HC03- ions are present so blood pH becomes alkaline...
Acid-base imbalances - compensation of respiratory acidosis and alkalosis
By: HWC, Views: 11949
• When one pH balancing system is affected then the other balancing system attempts to correct, or compensate for, the pH imbalance. - Respiratory acidosis: • Excessive CO2 is present so blood pH becomes acidic. • Compensation is increased secretion of H+ into urine and reabsorption ...
Role of the respiratory system - effect of altered ventilation rates
By: HWC, Views: 11601
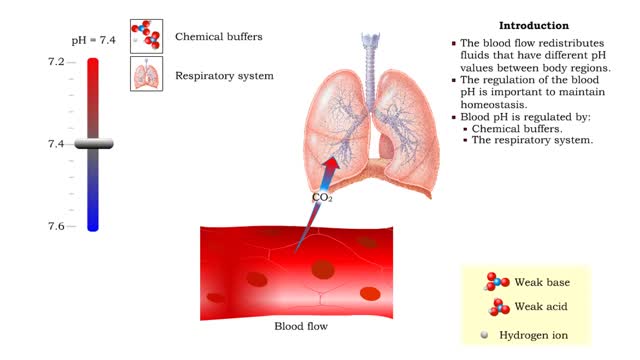
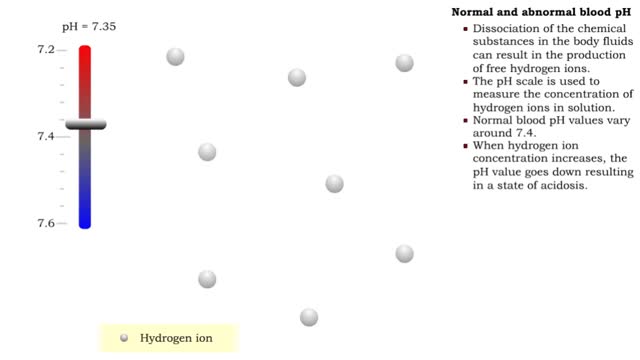
• Dissociation of the chemical substances in the body fluids can result in the production of free hydrogen ions. • The pH scale is used to measure the concentration of hydrogen ions in solution. • Normal blood pH values vary around 7.4. • When hydrogen ion concentration increases, t...
By: HWC, Views: 11799
• Dissociation of the chemical substances in the body fluids can result in the production of free hydrogen ions. • The pH scale is used to measure the concentration of hydrogen ions in solution. • Normal blood pH values vary around 7.4. • When hydrogen ion concentration increases, t...
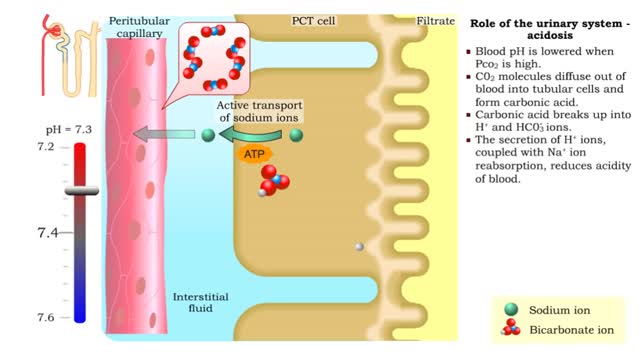
Role of the urinary system - acidosis and alkalosis
By: HWC, Views: 11922
• Tubular cells of the proximal convoluted tubule and collecting tubules can alter filtrate pH and therefore blood pH. • These cells can affect blood pH with two coupled mechanisms: • Reabsorption of bicarbonate ions. • Secretion of hydrogen ions. • The reabsorption of bicarbonate...
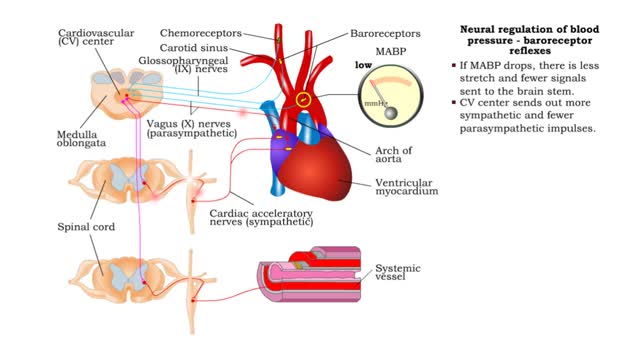
Neural regulation of blood pressure - baroreceptor and chemoreceptor reflexes
By: HWC, Views: 12079
• The nervous system regulates blood pressure with two reflex arcs: baroreceptor and chemoreceptor. ■ Baroreceptors (pressure) and chemoreceptors (chemical) are located in the carotid sinus and aortic arch. • Carotid sinus reflex helps maintain normal blood pressure in brain. • Ba...
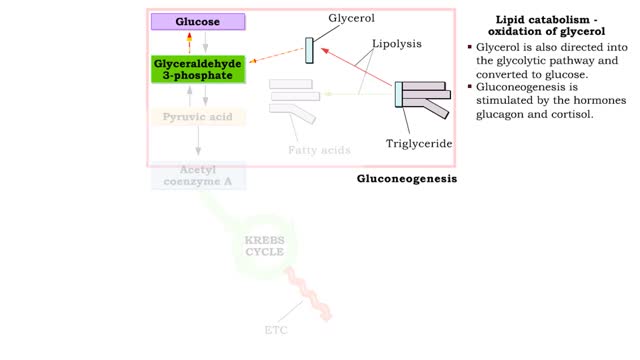
Lipid catabolism ( ketogenesis and oxidation of glycerol) and Lipid anabolism (lipogenesis)
By: HWC, Views: 12067
• During excessive beta oxidation, the two-carbon fatty acid fragments are converted into acidic ketone bodies. • Ketosis, the overproduction of ketone bodies, can lead to acidosis (ketoacidosis) of the blood. • After lipolysis, glycerol is converted to pyruvic acid. • Pyruvic aci...
Advertisement