Search Results
Results for: 'hypertonic%20solution'
By: HWC, Views: 8603
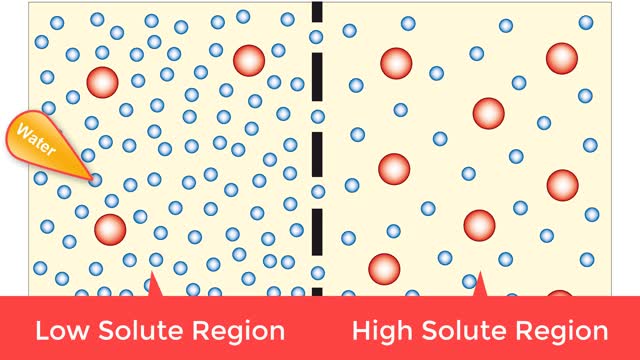
Osmosis is when a solvent, such as water, moves from a low-solute concentration solution to a higher-solute concentration solution through a semipermeable. Osmosis is an example of diffusion (a special case of diffusion) in which the molecules are water, and the concentration gradient occurs a...
By: HWC, Views: 8095
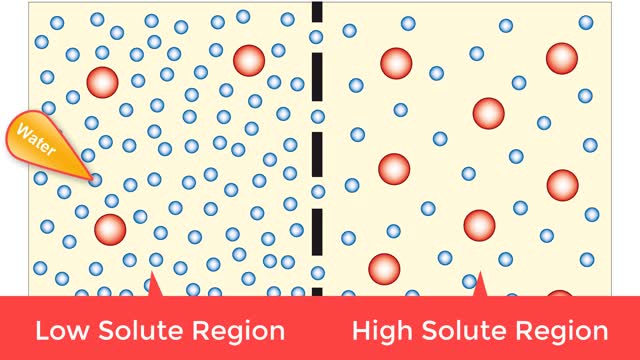
Osmosis is when a solvent, such as water, moves from a low-solute concentration solution to a higher-solute concentration solution through a semipermeable. Osmosis is an example of diffusion (a special case of diffusion) in which the molecules are water, and the concentration gradient occurs a...
Osmosis - Isotonic, Hypotonic, and Hypertonic Solutions
By: HWC, Views: 11011
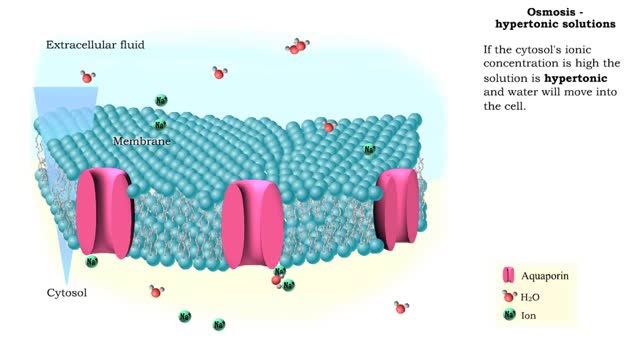
Isotonic: Equal Water moves in and out of the cell at an equal rate. The cell remains unchanged. Hypotonic: "hypo" hippo Water moves into the cell, making it swell and get fat (like a hippo). Eventually the cell can rupture and burst (aka lyse). Hypertonic: "like a raisin" Water leaves...
Advertisement