Search Results
Results for: 'water retention'
Non-polar compounds - insolubility
By: HWC, Views: 11103
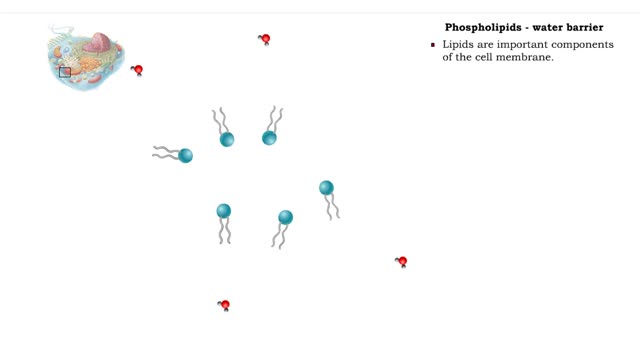
• A non-polar molecule has uniform distribution of electrons. • Non-polar compounds like fatty acids in lipids have a high proportion of carbon and hydrogen. • Lipids possess no charge or partial charge. • Lipids are not attracted to water molecules. • Lipids are not soluble in...
Properties of water -structure of water and polarity (Ionized and polar compounds)
By: HWC, Views: 11123
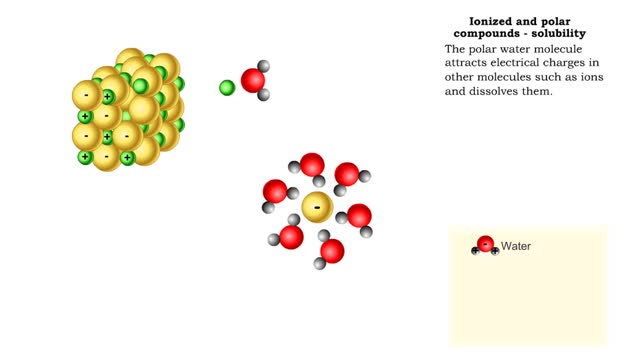
■ Water transports most of the molecules in the body. ■ The structure of a water molecule allows it to dissolve other molecules. ■ Shared electrons spend more time near the oxygen atom. ■ Oxygen end has a partial negative charge. ■ Hydrogen ends have a partial positive charge....
Osmosis - Isotonic, Hypotonic, and Hypertonic Solutions
By: HWC, Views: 11082
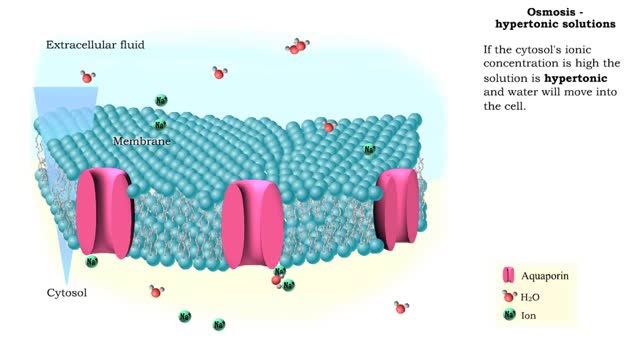
Isotonic: Equal Water moves in and out of the cell at an equal rate. The cell remains unchanged. Hypotonic: "hypo" hippo Water moves into the cell, making it swell and get fat (like a hippo). Eventually the cell can rupture and burst (aka lyse). Hypertonic: "like a raisin" Water leaves...
By: HWC, Views: 8671
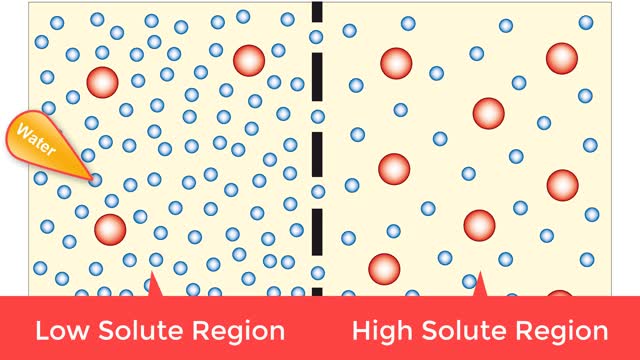
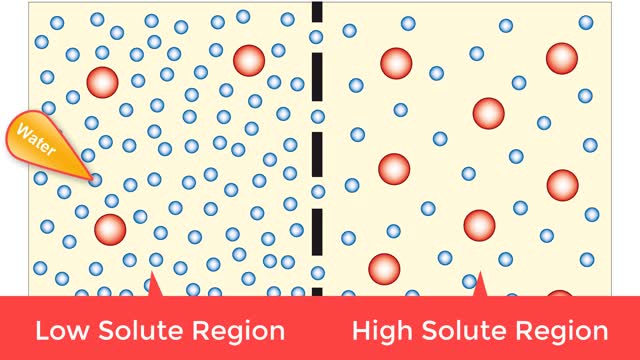
Osmosis is when a solvent, such as water, moves from a low-solute concentration solution to a higher-solute concentration solution through a semipermeable. Osmosis is an example of diffusion (a special case of diffusion) in which the molecules are water, and the concentration gradient occurs a...
By: HWC, Views: 8166
Osmosis is when a solvent, such as water, moves from a low-solute concentration solution to a higher-solute concentration solution through a semipermeable. Osmosis is an example of diffusion (a special case of diffusion) in which the molecules are water, and the concentration gradient occurs a...
What are Strong & Weak Acids and How they're different?
By: HWC, Views: 9769
Let's consider the changes that take place when hydrogen chloride, HCI, is added to water. You will need to recognize space-filling models of HCI molecules, hydronium ions (H30+), chloride ions (C11, and water molecules (H20). They are shown at the right. When HC1 molecules dissolve in water, ...
By: HWC, Views: 10278
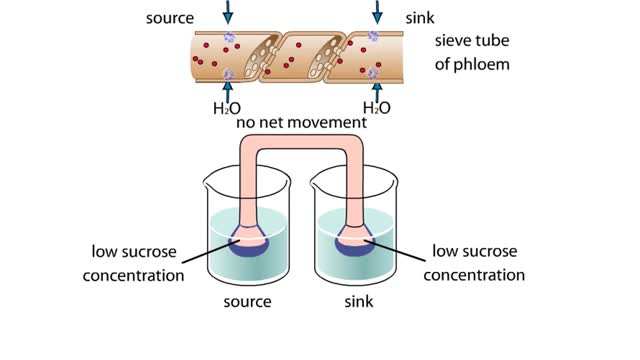
This apparatus of beakers A and funnels simulates the flow of a sucrose solution in the phloem of a plant. The funnels and connecting tube represent a sieve tube of the phloem. Differentially permeable membranes cap the funnels at the source and sink ends, allowing water, but not sucrose, to cros...
Forming urine ( influencing factors), Forming dilute urine & Forming concentrated urine
By: HWC, Views: 11476
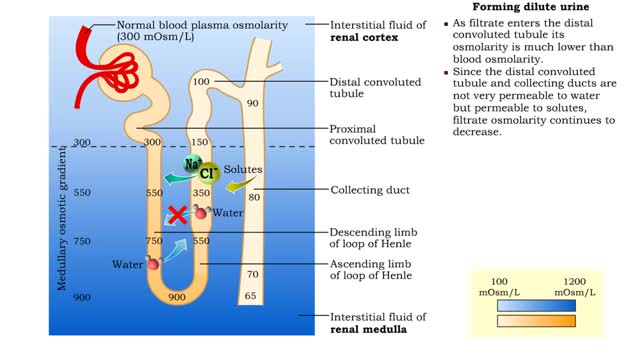
• The amount of urine produced by the nephron depends on : • Body fluid volume. • Body fluid composition. • Dilute urine is formed when the body is normally hydrated. • The medullary osmotic gradient determines the osmolarity of the filtrate. • Filtrate osmolarity increase...
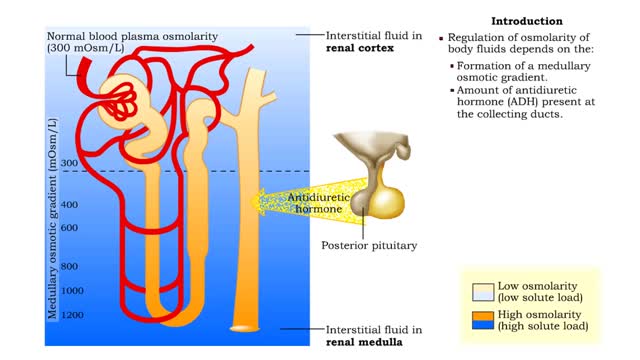
Medullary osmotic gradient - influencing factors
By: HWC, Views: 11449
▪ Maintenance of fluid volume and composition, despite changes in water input and output, is crucial to a healthy life. ▪ Regulation of blood's osmolarity, or solute concentration, is a function of the nephron. • Normal osmolarity is maintained by the ability of the nephron to alter uri...
Advertisement