Search Results
Results for: 'Hydrogen bonds'
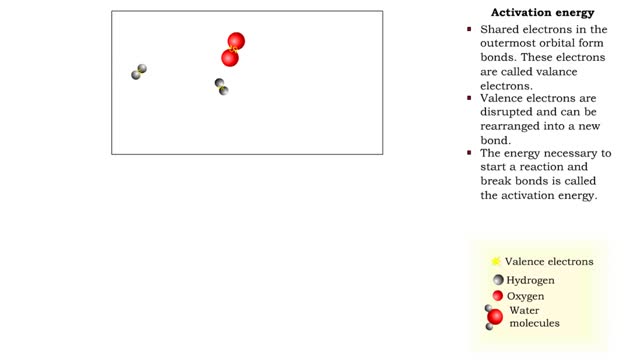
Activation Energy - Valence Electrons
By: HWC, Views: 9999
■ Shared electrons in the outermost orbital form bonds. These electrons are called valence electrons. ■ Valence electrons are disrupted and can be rearranged into a new bond. ■ The energy necessary to start a reaction and break bonds is called the activation energy. ■ Reactants have...
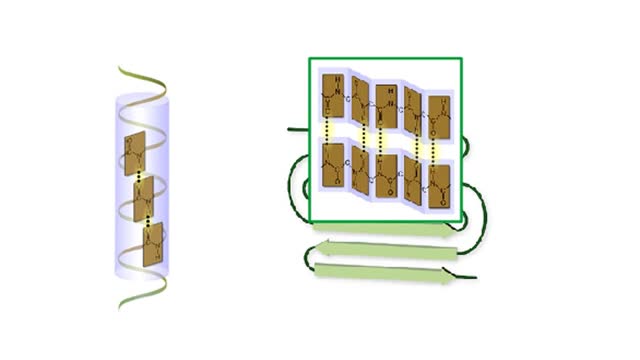
Protein Secondary and Tertiary Structures - Animation
By: HWC, Views: 6721
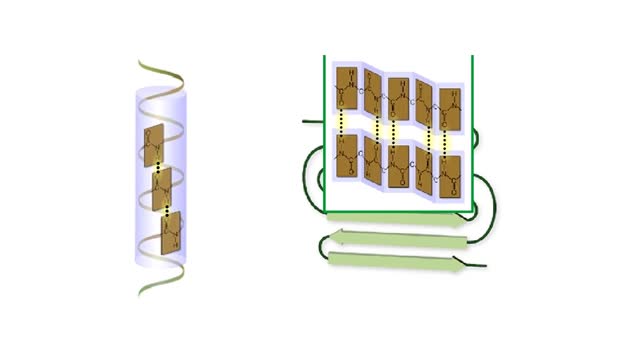
Amino acid sequence dictates a protein's final shape. The presence of certain amino acids favors a pattern of hydrogen bonding that causes part of the polypeptide chain to coil and twist into an alpha helix. The presence of other amino acids enables hydrogen bonding between strand like r...
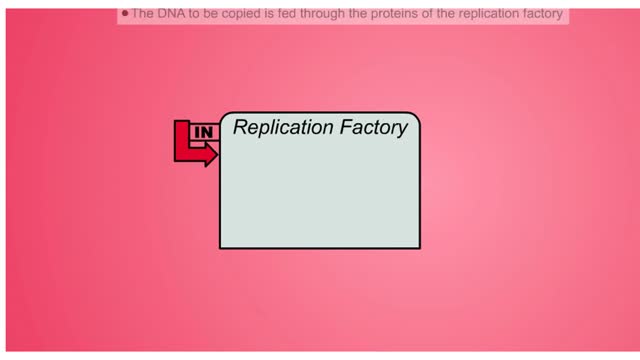
DNA Replication Factory and Protein
By: HWC, Views: 10113
DNA (deoxyribose nucleic acid) carries all the genetic information needed to re-create itself and to pass on the characteristics of the organism. The “factory” model of DNA replication hypothesizes a specific nuclear structure in which the molecular machinery for replication forks are brou...
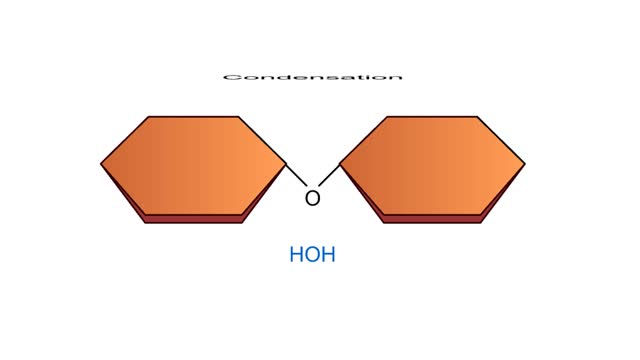
Condensation and Hydrolysis Animation
By: HWC, Views: 4371
A condensation reaction joins two molecules together to form one larger molecule. An enzyme removes a hydroxyl group from one molecule and a hydrogen atom from another, then speeds the formation of a bond between the two molecules at their exposed sites. Typically the discarded atoms join t...
By: HWC, Views: 10443
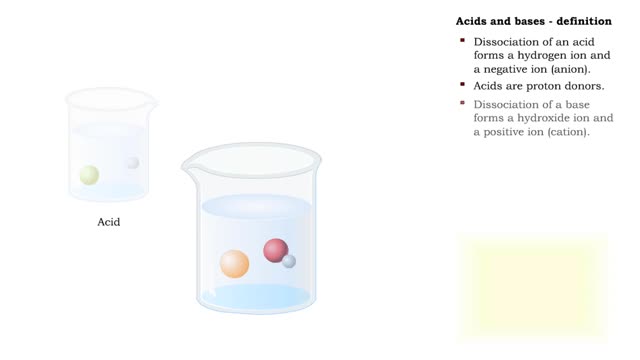
Acids and bases are found all around your house. For example, if you open your pantry or refrigerator, you might see a lot of acids. Fruit juice, soda pop, vinegar, and milk are all examples of acids. The word acid actually comes from a Latin term meaning ''sour.'' Many materials, like sugar for ...
Secondary and tertiary levels of protein structure Animation
By: HWC, Views: 4411
Amino acid sequence dictates a protein's final shape. The presence of certain amino acids favors a pattern of hydrogen bonding that causes part of the polypeptide chain to coil and twist into an alpha helix. The presence of other amino acids enables hydrogen bonding between strand like re...
Role of the respiratory system - effect of altered ventilation rates
By: HWC, Views: 10375
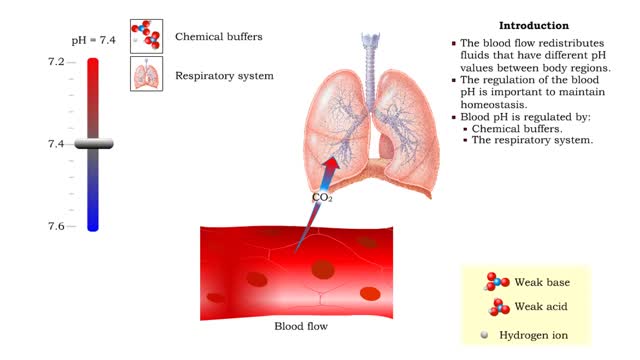
• Dissociation of the chemical substances in the body fluids can result in the production of free hydrogen ions. • The pH scale is used to measure the concentration of hydrogen ions in solution. • Normal blood pH values vary around 7.4. • When hydrogen ion concentration increases, t...
Role of the urinary system - acidosis and alkalosis
By: HWC, Views: 10681
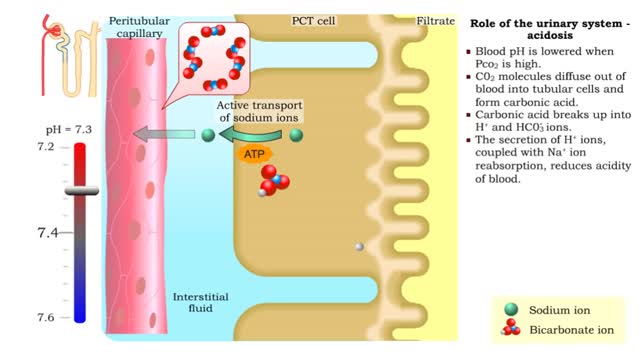
• Tubular cells of the proximal convoluted tubule and collecting tubules can alter filtrate pH and therefore blood pH. • These cells can affect blood pH with two coupled mechanisms: • Reabsorption of bicarbonate ions. • Secretion of hydrogen ions. • The reabsorption of bicarbonate...
Digestive chemicals - water, gastric acid, bile & bicarbonate
By: HWC, Views: 10263
• Water is the most abundant molecule in ingested fluids. • Water plays a primary role in hydrolytic digestive reactions. • Helps liquefy and transport digestive foodstuffs down the tract. • Transports secretions from accessory digestive organs to gastrointestinal tract. • Aids ...
Advertisement