Primary Active Transport - electrochemical gradient and ion transport / water movement
Energy derived from ATP changes the shape of a transporter protein which pumps a substance across a plasma membrane against its concentration gradient An electrochemical gradient is a gradient of electrochemical potential, usually for an ion that can move across a membrane. The gradient consists of two parts, the chemical gradient, or difference in solute concentration across a membrane, and the electrical gradient, or difference in charge across a membrane. When there are unequal concentrations of an ion across a permeable membrane, the ion will move across the membrane from the area of higher concentration to the area of lower concentration through simple diffusion. Ions also carry an electric charge that forms an electric potential across a membrane. If there is an unequal distribution of charges across the membrane, then the difference in electric potential generates a force that drives ion diffusion until the charges are balanced on both sides of the membrane. An electrochemical gradient has two components. First, the electrical component is caused by a charge difference across the lipid membrane. Second, a chemical component is caused by a differential concentration of ions across the membrane. The combination of these two factors determines the thermodynamically favorable direction for an ion's movement across a membrane. Ions are moved across the membrane to determine a solutions ionic concentration. Water moves via osmosis towards a higher ionic concentration.
Add To
You must login to add videos to your playlists.
Advertisement



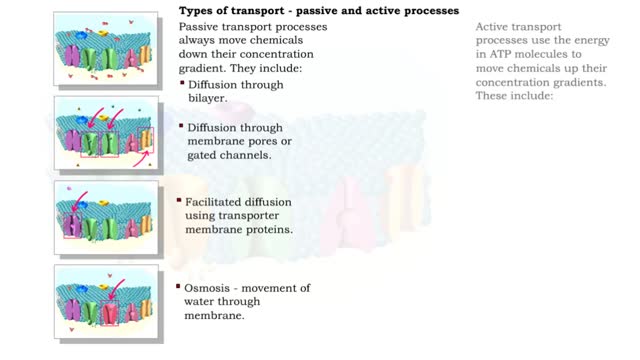
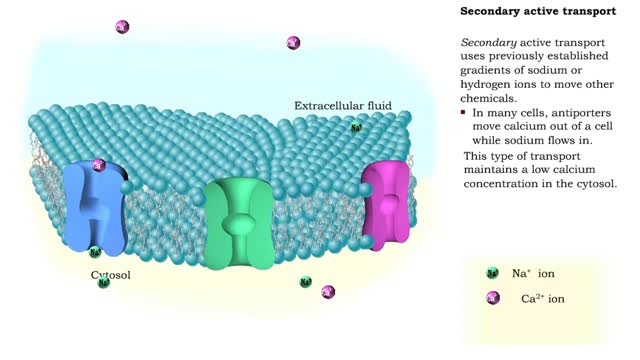
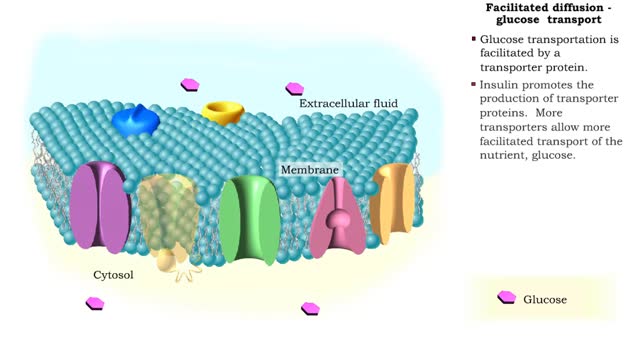
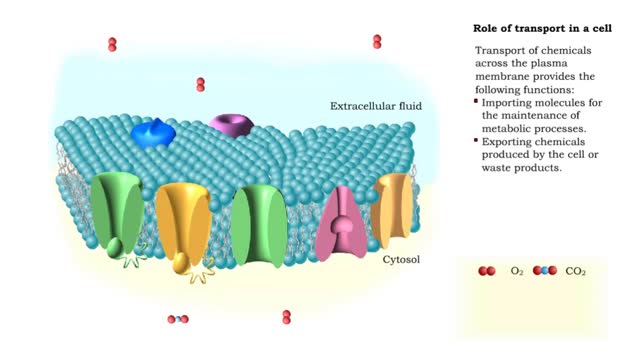
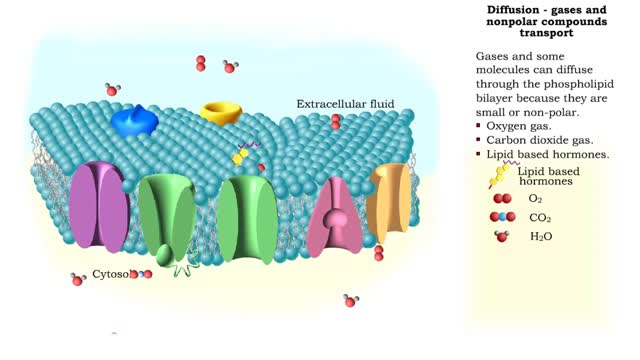
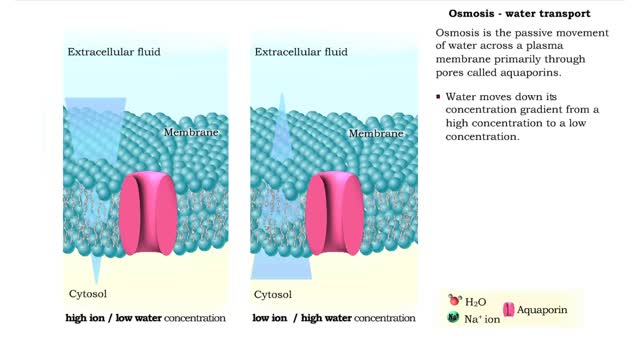
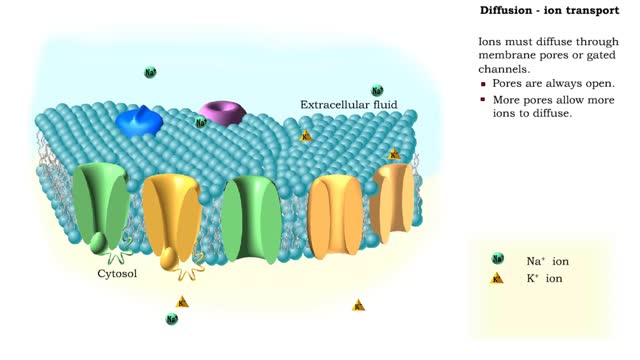
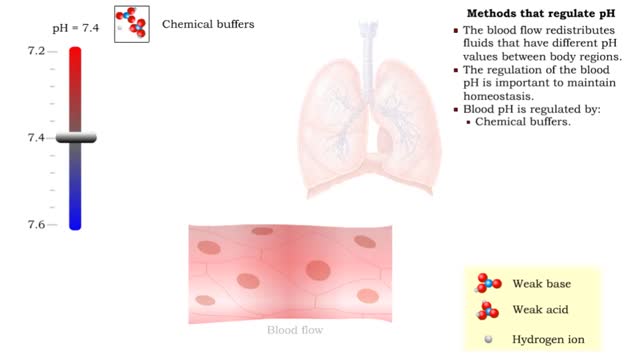
Comments
0 Comments total
Sign In to post comments.
No comments have been posted for this video yet.