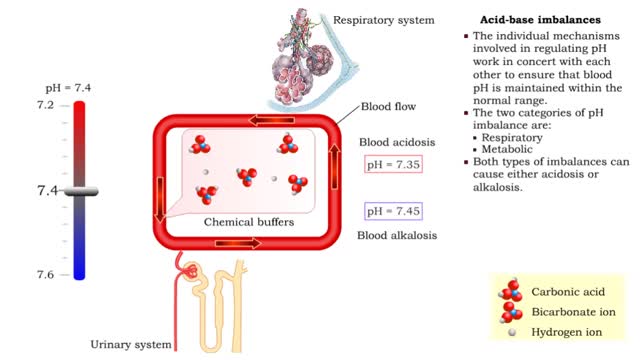
Normal and abnormal blood pH
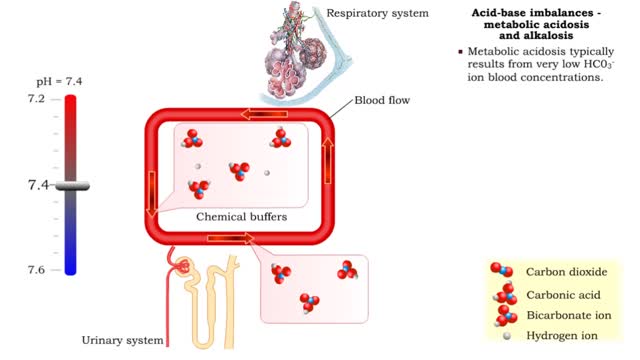
• Dissociation of the chemical substances in the body fluids can result in the production of free hydrogen ions. • The pH scale is used to measure the concentration of hydrogen ions in solution. • Normal blood pH values vary around 7.4. • When hydrogen ion concentration increases, the pH value goes down resulting in a state of acidosis. • When hydrogen ion concentration decreases, the pH value goes up resulting in a state of alkalosis. • Life sustaining chemical reactions are catalyzed by enzymes which can only function effectively within narrow pH ranges.
Add To
You must login to add videos to your playlists.
Advertisement



Comments
0 Comments total
Sign In to post comments.
No comments have been posted for this video yet.