Chemical Buffers - protein buffer, phosphate buffer system and bicarbonate buffer system
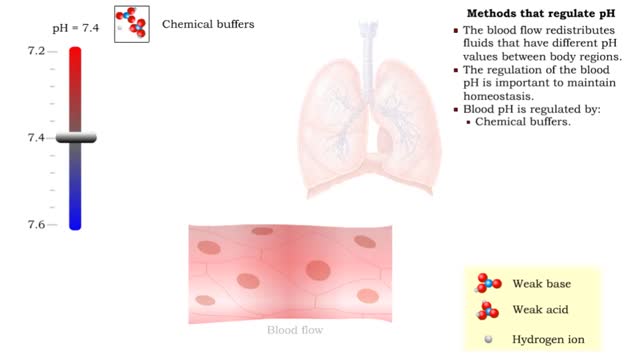
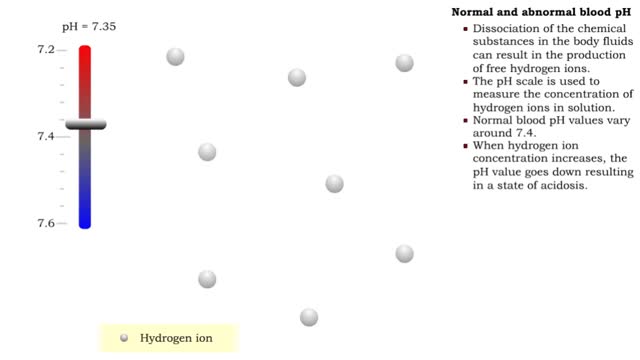
• There are a variety of chemicals in body fluids that prevent the fluids from undergoing large changes in. • These chemicals buffer or regulate fluctuations in H+ concentration. • Chemical buffers: • Bind to H+ ions when there are too many in a solution so pH remains normal. • Release H+ ions when there are too few in a solution so pH remains normal. • The chemical buffer systems include: • Protein system. • Phosphate system. • Carbonic acid-bicarbonate system. The protein buffer system is important in regulating blood plasma and intracellular fluid pH. • If plasma or extracellular fluid pH drops due to excessive H+ ions, then H+ ions bind to amine groups and pH rises. • If plasma or extracellular fluid pH rises due to a shortage of H+ ions, then acid groups dissociate and pH drops. The phosphate system is most important in regulating intracellular fluid pH. • If intracellular fluid pH drops due to excessive H+ ions, then dihydrogen phosphate is formed and intracellular fluid pH rises. • If intracellular fluid pH rises due to a shortage of H+ ions, then monohydrogen phosphate ions are formed and intracellular fluid pH. The carbonic acid-bicarbonate system is most important in regulating blood plasma pH. • If plasma pH drops due to excessive H+ ions, then carbonic acid is formed and plasma pH rises. • If plasma pH rises due to a shortage of H+ ions, then bicarbonate ions are formed and plasma pH drops.
Add To
You must login to add videos to your playlists.
Advertisement



Comments
0 Comments total
Sign In to post comments.
No comments have been posted for this video yet.