Search Results
Results for: 'Bicarbonate'
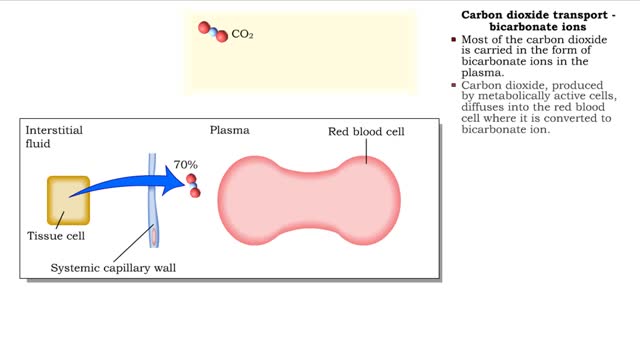
Methods of carbon dioxide transport - carbaminohemoglobin and bicarbonate ions
By: HWC, Views: 11775
• Carbon dioxide is transported three ways: • As bicarbonate ions in the plasma. • Bound to hemoglobin. • As a dissolved gas in the plasma. • A small percent of carbon dioxide is transported as a dissolved gas. • Some of the carbon dioxide is bound to hemoglobin, in the fo...
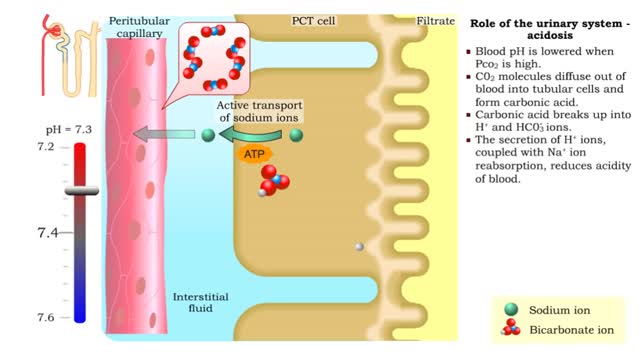
Role of the urinary system - acidosis and alkalosis
By: HWC, Views: 11929
• Tubular cells of the proximal convoluted tubule and collecting tubules can alter filtrate pH and therefore blood pH. • These cells can affect blood pH with two coupled mechanisms: • Reabsorption of bicarbonate ions. • Secretion of hydrogen ions. • The reabsorption of bicarbonate...
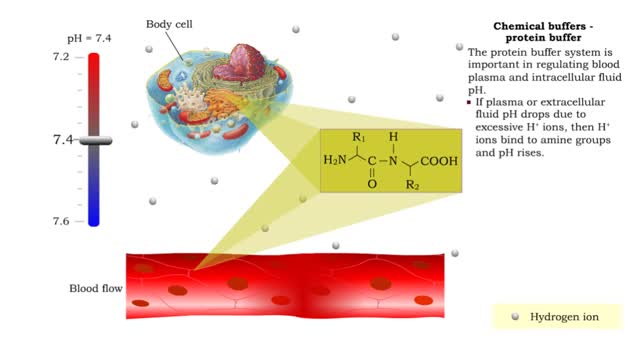
Chemical Buffers - protein buffer, phosphate buffer system and bicarbonate buffer system
By: HWC, Views: 11929
• There are a variety of chemicals in body fluids that prevent the fluids from undergoing large changes in. • These chemicals buffer or regulate fluctuations in H+ concentration. • Chemical buffers: • Bind to H+ ions when there are too many in a solution so pH remains normal. •...
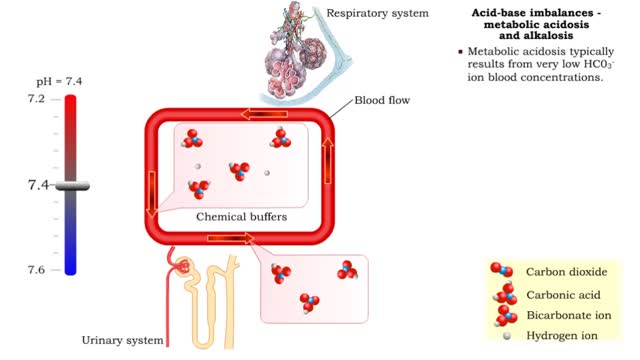
Acid-base imbalances - compensation of respiratory acidosis and alkalosis
By: HWC, Views: 11959
• When one pH balancing system is affected then the other balancing system attempts to correct, or compensate for, the pH imbalance. - Respiratory acidosis: • Excessive CO2 is present so blood pH becomes acidic. • Compensation is increased secretion of H+ into urine and reabsorption ...
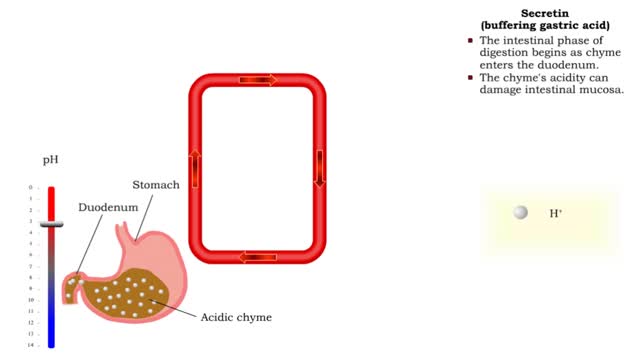
Gastrin (gastric emptying) & Secretin (buffering gastric acid)
By: HWC, Views: 11254
• Gastrin also binds to the smooth muscle cells in the stomach causing: • Increased gastric motility. • Opening of pyloric sphincter. • Increased gastric emptying. • The intestinal phase of digestion begins as chyme enters the duodenum. • The chyme's acidity can damage int...
Digestive chemicals - water, gastric acid, bile & bicarbonate
By: HWC, Views: 11476
• Water is the most abundant molecule in ingested fluids. • Water plays a primary role in hydrolytic digestive reactions. • Helps liquefy and transport digestive foodstuffs down the tract. • Transports secretions from accessory digestive organs to gastrointestinal tract. • Aids ...
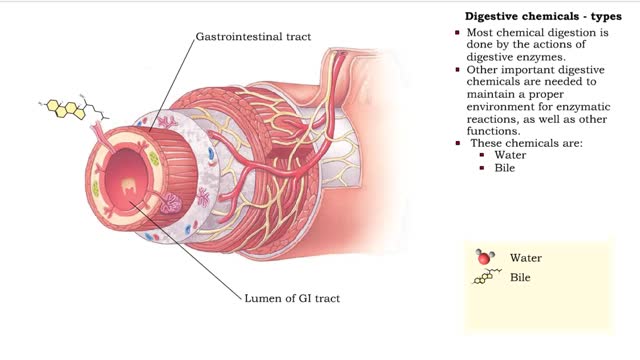
Digestive chemicals - types & enzymes
By: HWC, Views: 11780
• Chemical digestion breaks down food as it moves through the digestive tract. • Using enzymes and other digestive chemicals, the process reduces food particles into nutrient molecules that can be absorbed. • Most chemical digestion is done by the actions of digestive enzymes. • O...
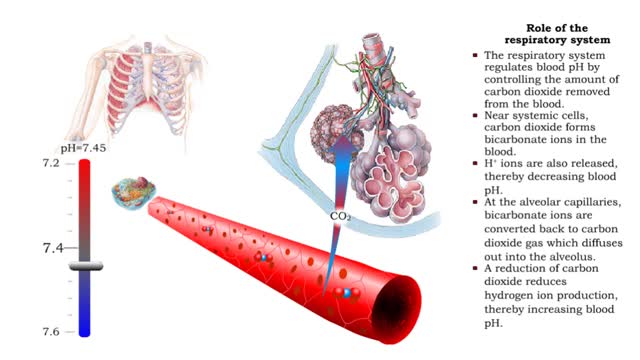
Role of the respiratory system - effect of altered ventilation rates
By: HWC, Views: 12318
• The respiratory system regulates blood pH by controlling the amount of carbon dioxide removed from the blood. • Near systemic cells, carbon dioxide forms bicarbonate ions in the blood. H+ ions are also released, thereby decreasing blood pH. • At the alveolar capillaries, bicarbonate io...
Role of the respiratory system - effect of altered ventilation rates
By: HWC, Views: 11609
• Dissociation of the chemical substances in the body fluids can result in the production of free hydrogen ions. • The pH scale is used to measure the concentration of hydrogen ions in solution. • Normal blood pH values vary around 7.4. • When hydrogen ion concentration increases, t...
Advertisement