Search Results
Results for: 'Buffer solution'
Buffers definition and the role of buffer in the body
By: HWC, Views: 11162
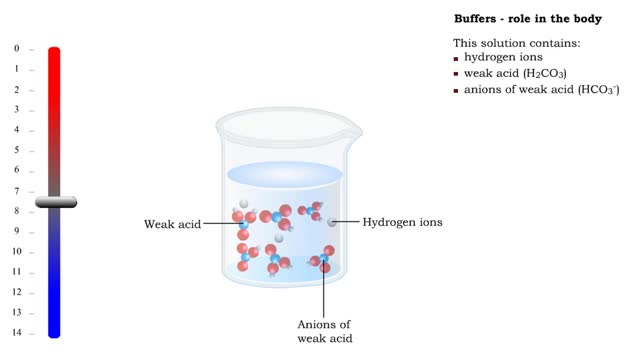
■ Too many H+ break hydrogen bonds and a protein comes apart. ■ Buffers react with excess H+ to protect proteins from breaking down. ■ Buffers consist of weak acid plus anions of that weak acid. This solution contains: • hydrogen ions • weak acid (H2CO3) • anions of we...
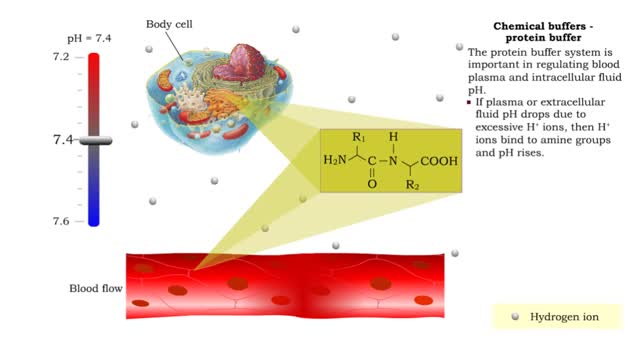
Chemical Buffers - protein buffer, phosphate buffer system and bicarbonate buffer system
By: HWC, Views: 11303
• There are a variety of chemicals in body fluids that prevent the fluids from undergoing large changes in. • These chemicals buffer or regulate fluctuations in H+ concentration. • Chemical buffers: • Bind to H+ ions when there are too many in a solution so pH remains normal. •...
By: HWC, Views: 8291
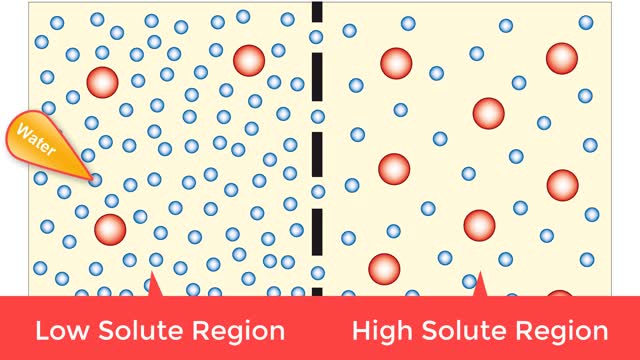
Osmosis is when a solvent, such as water, moves from a low-solute concentration solution to a higher-solute concentration solution through a semipermeable. Osmosis is an example of diffusion (a special case of diffusion) in which the molecules are water, and the concentration gradient occurs a...
By: HWC, Views: 8815
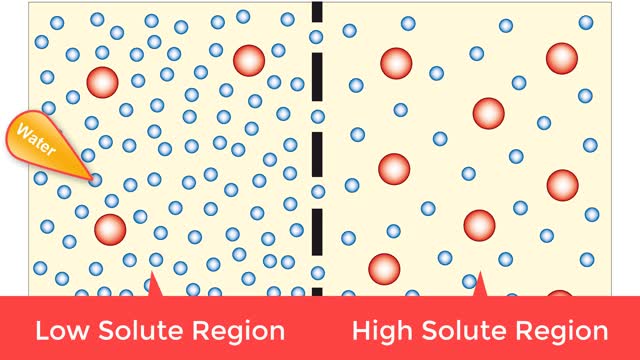
Osmosis is when a solvent, such as water, moves from a low-solute concentration solution to a higher-solute concentration solution through a semipermeable. Osmosis is an example of diffusion (a special case of diffusion) in which the molecules are water, and the concentration gradient occurs a...
Double Vacuum Process (Illustration No Audio)
By: HWC, Views: 10169
In this so-called low-pressure process, the wood is first subjected to a short and relatively weak initial vacuum, after which the treatment vessel is flooded with preservative solution and reduced to normal pressure The double vacuum container is loaded with timber. A partial vacuum is draw...
What are Strong & Weak Acids and How they're different?
By: HWC, Views: 9906
Let's consider the changes that take place when hydrogen chloride, HCI, is added to water. You will need to recognize space-filling models of HCI molecules, hydronium ions (H30+), chloride ions (C11, and water molecules (H20). They are shown at the right. When HC1 molecules dissolve in water, ...
By: HWC, Views: 10992
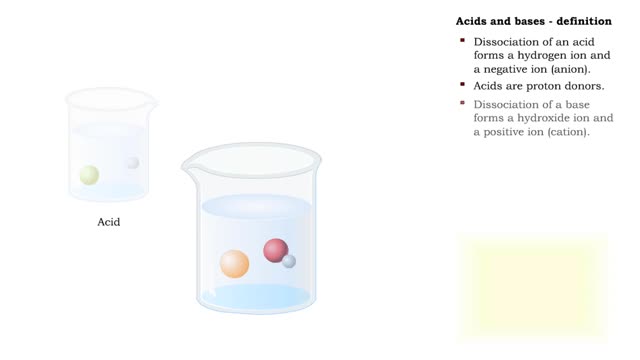
Acids and bases are found all around your house. For example, if you open your pantry or refrigerator, you might see a lot of acids. Fruit juice, soda pop, vinegar, and milk are all examples of acids. The word acid actually comes from a Latin term meaning ''sour.'' Many materials, like sugar for ...
By: HWC, Views: 11277
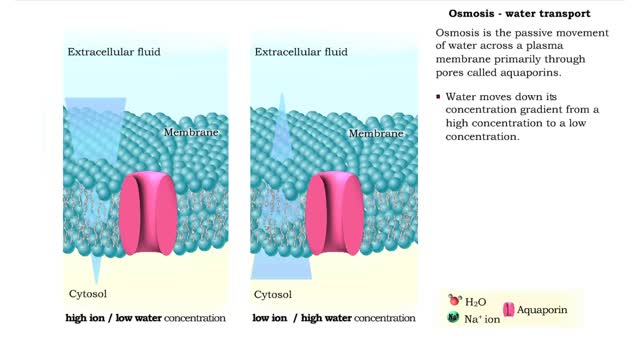
Osmosis is the flow of water down its concentration gradient, across a semi-permeable membrane. Osmosis is an example of diffusion, which is when molecules tend to distribute themselves evenly in a space. what is a semi-permeable membrane? It is a membrane or barrier that allows some molec...
By: HWC, Views: 10389
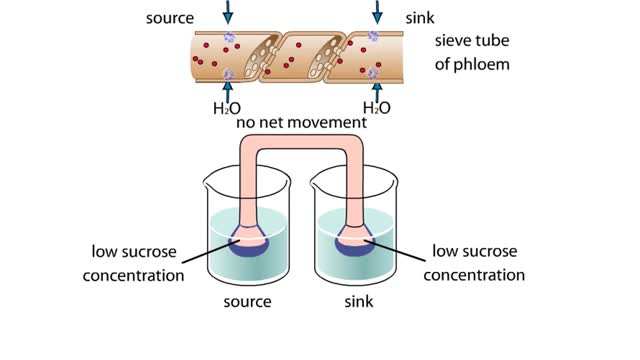
This apparatus of beakers A and funnels simulates the flow of a sucrose solution in the phloem of a plant. The funnels and connecting tube represent a sieve tube of the phloem. Differentially permeable membranes cap the funnels at the source and sink ends, allowing water, but not sucrose, to cros...
Advertisement