Search Results
Results for: 'Buffers'
Buffers definition and the role of buffer in the body
By: HWC, Views: 11162
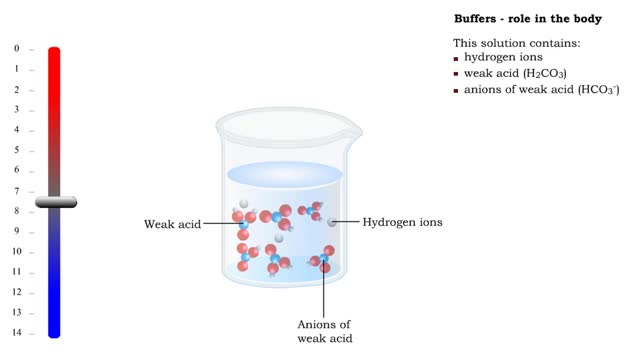
■ Too many H+ break hydrogen bonds and a protein comes apart. ■ Buffers react with excess H+ to protect proteins from breaking down. ■ Buffers consist of weak acid plus anions of that weak acid. This solution contains: • hydrogen ions • weak acid (H2CO3) • anions of we...
By: HWC, Views: 11234
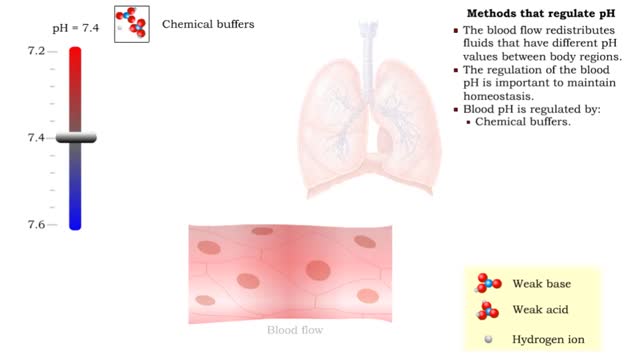
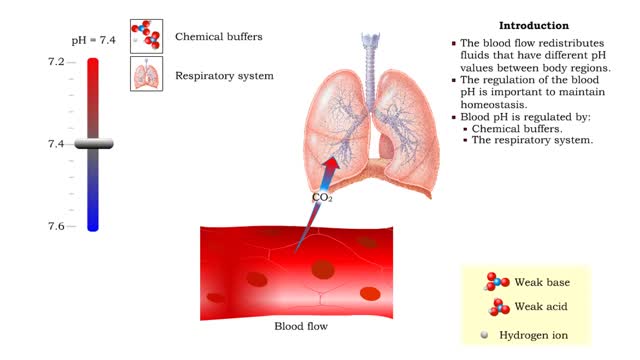
• The blood flow redistributes fluids that have different pH values between body regions. • The regulation of the blood pH is important to maintain homeostasis. • Blood pH is regulated by: • Chemical buffers. • The respiratory system. • The urinary system. • All thes...
Role of the respiratory system - effect of altered ventilation rates
By: HWC, Views: 11014
• Dissociation of the chemical substances in the body fluids can result in the production of free hydrogen ions. • The pH scale is used to measure the concentration of hydrogen ions in solution. • Normal blood pH values vary around 7.4. • When hydrogen ion concentration increases, t...
Chemical Buffers - protein buffer, phosphate buffer system and bicarbonate buffer system
By: HWC, Views: 11304
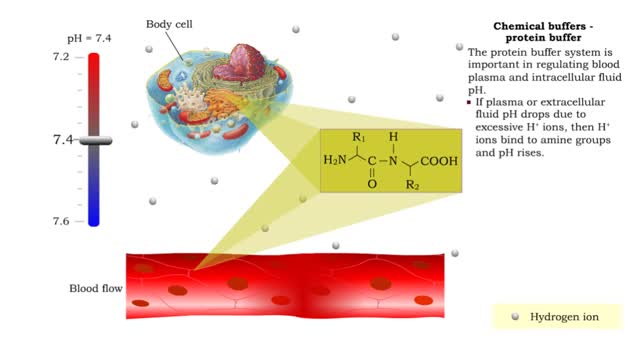
• There are a variety of chemicals in body fluids that prevent the fluids from undergoing large changes in. • These chemicals buffer or regulate fluctuations in H+ concentration. • Chemical buffers: • Bind to H+ ions when there are too many in a solution so pH remains normal. •...
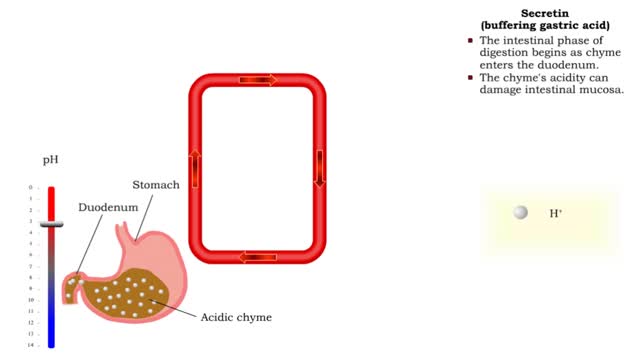
Gastrin (gastric emptying) & Secretin (buffering gastric acid)
By: HWC, Views: 10676
• Gastrin also binds to the smooth muscle cells in the stomach causing: • Increased gastric motility. • Opening of pyloric sphincter. • Increased gastric emptying. • The intestinal phase of digestion begins as chyme enters the duodenum. • The chyme's acidity can damage int...
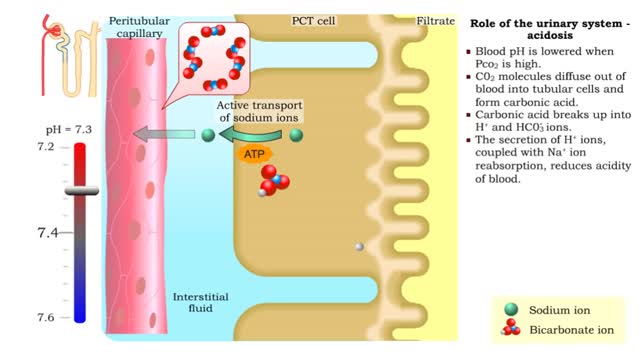
Role of the urinary system - acidosis and alkalosis
By: HWC, Views: 11263
• Tubular cells of the proximal convoluted tubule and collecting tubules can alter filtrate pH and therefore blood pH. • These cells can affect blood pH with two coupled mechanisms: • Reabsorption of bicarbonate ions. • Secretion of hydrogen ions. • The reabsorption of bicarbonate...
Advertisement