Search Results
Results for: 'Compare Osmosis with Reverse Osmosis'
By: HWC, Views: 8289
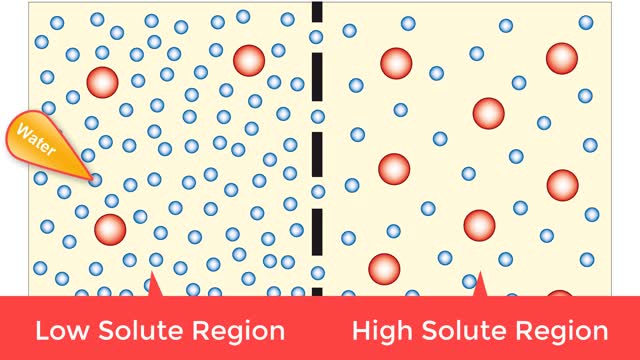
Osmosis is when a solvent, such as water, moves from a low-solute concentration solution to a higher-solute concentration solution through a semipermeable. Osmosis is an example of diffusion (a special case of diffusion) in which the molecules are water, and the concentration gradient occurs a...
By: HWC, Views: 8810
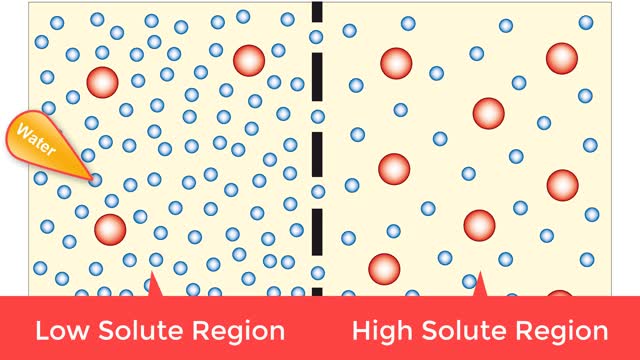
Osmosis is when a solvent, such as water, moves from a low-solute concentration solution to a higher-solute concentration solution through a semipermeable. Osmosis is an example of diffusion (a special case of diffusion) in which the molecules are water, and the concentration gradient occurs a...
By: HWC, Views: 11272
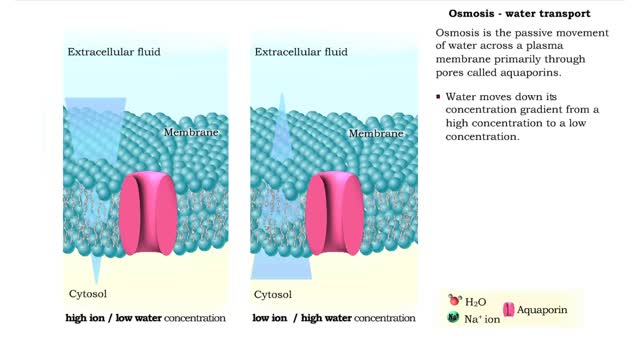
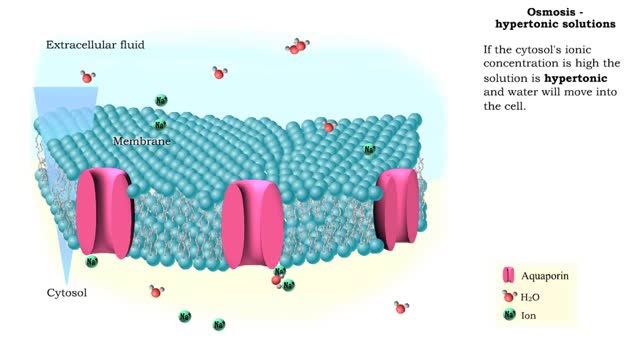
Osmosis is the flow of water down its concentration gradient, across a semi-permeable membrane. Osmosis is an example of diffusion, which is when molecules tend to distribute themselves evenly in a space. what is a semi-permeable membrane? It is a membrane or barrier that allows some molec...
Type of Transport - Active and Passive Processes
By: HWC, Views: 11435
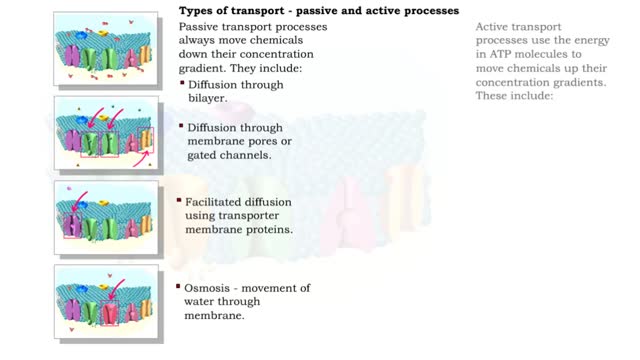
Active transport moves materials from lower to a higher concentration, while passive transport moves materials from higher to lower concentration. Active transport requires energy to proceed, while passive transport does not require the input of extra energy to occur. Transport processes that ...
Osmosis - Isotonic, Hypotonic, and Hypertonic Solutions
By: HWC, Views: 11222
Isotonic: Equal Water moves in and out of the cell at an equal rate. The cell remains unchanged. Hypotonic: "hypo" hippo Water moves into the cell, making it swell and get fat (like a hippo). Eventually the cell can rupture and burst (aka lyse). Hypertonic: "like a raisin" Water leaves...
By: HWC, Views: 10383
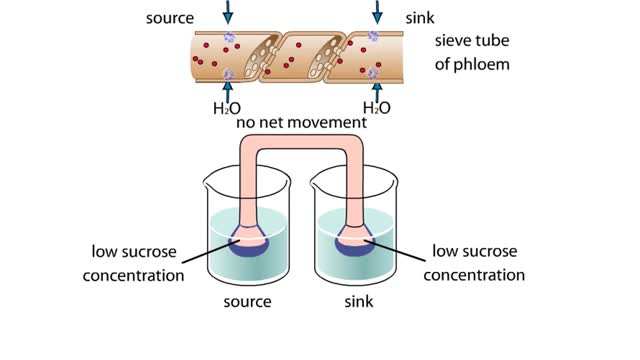
This apparatus of beakers A and funnels simulates the flow of a sucrose solution in the phloem of a plant. The funnels and connecting tube represent a sieve tube of the phloem. Differentially permeable membranes cap the funnels at the source and sink ends, allowing water, but not sucrose, to cros...
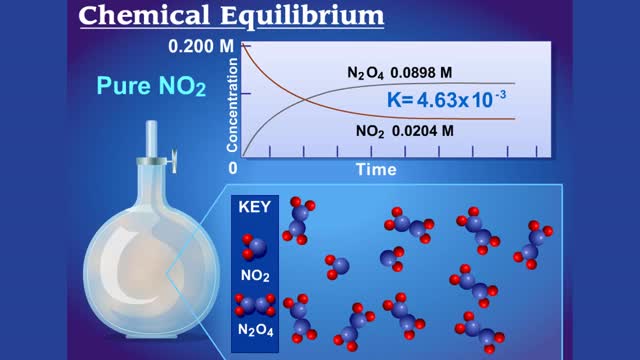
Chemical Equilibrium between N2O4 (colorless gas) and NO2 (brown gas)
By: HWC, Views: 10330
For a system at equilibrium: ◆ both forward and reverse reactions are occurring simultaneously ◆ rate of forward reaction must equal rate of reverse reaction OR Rate forward = Rate reverse ◆ concentrations of reactants and products remain constant with time the equilibrium positio...
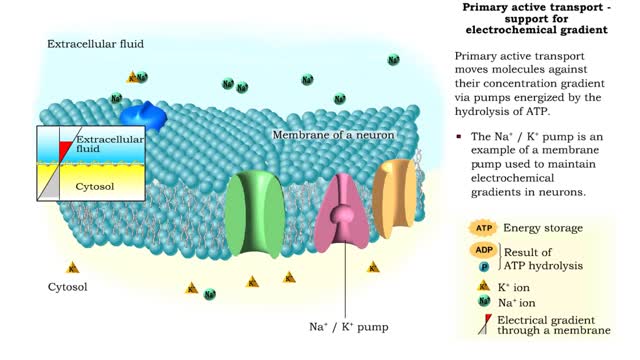
Primary Active Transport - electrochemical gradient and ion transport / water movement
By: HWC, Views: 11304
Energy derived from ATP changes the shape of a transporter protein which pumps a substance across a plasma membrane against its concentration gradient An electrochemical gradient is a gradient of electrochemical potential, usually for an ion that can move across a membrane. The gradient consis...
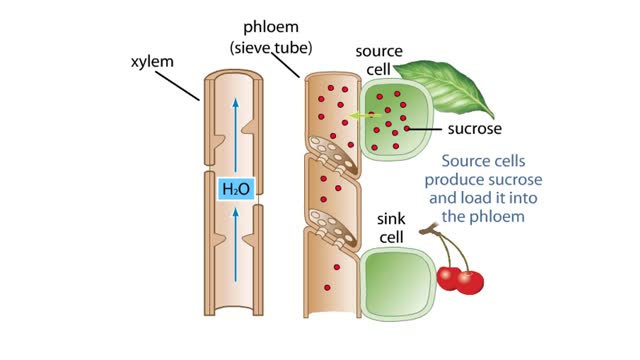
The Pressure Flow Model in a Plant
By: HWC, Views: 10506
The vascular system of plants has two transport tissues, called xylem and phloem. Xylem transports water and minerals, while phloem transports a variety of dissolved substances, including sugars and amino acids, throughout the plant. Water in the xylem always moves up, in the direction from th...
Advertisement