Search Results
Results for: 'nonpolar'
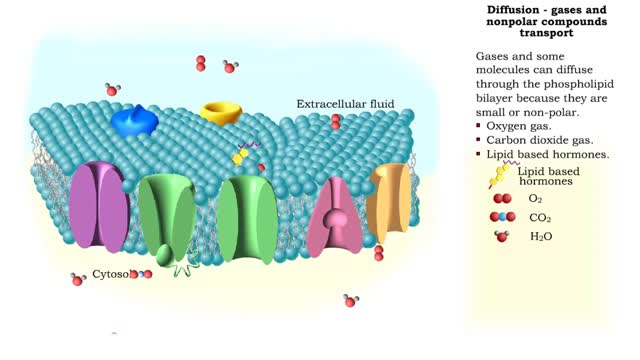
Simple Diffusion - gases and nonpolar compounds transport
By: HWC, Views: 12220
Gases and some molecules can diffuse through the phospholipid bilayer because they are small or non-polar. Oxygen gas. Carbon dioxide gas. Lipid based hormones. Plasma membranes are selectively permeable: The lipid bilayer is always permeable to small, nonpolar, uncharged molecules ...
By: HWC, Views: 11242
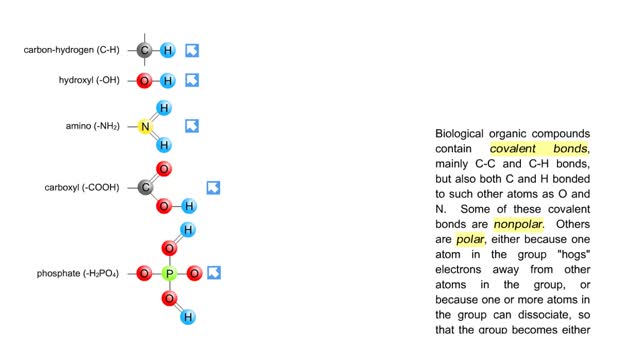
Biological organic compounds contain covalent bonds, mainly C-C and C-H bonds, but also both C and H bonded to such other atoms as O and N. Some of these covalent bonds are nonpolar. Others are polar, either because one atom in the group "hogs" electrons away from other atoms in the group, or...
Bond in biological molecules (Ionic, Covalent and Hydrogen bonds)/ How atoms bond?
By: HWC, Views: 8996
Sodium atoms and chloride atoms have unfilled orbitals in their outer shells. The lone electron in the outermost shell of a sodium atom can be pulled or knocked out. This ionizes the atom. It is now a positively charged sodium ion. A chlorine atom has an electron vacancy in its outer shell and...
Major Elements in Biological Molecules: Lipids
By: HWC, Views: 11108
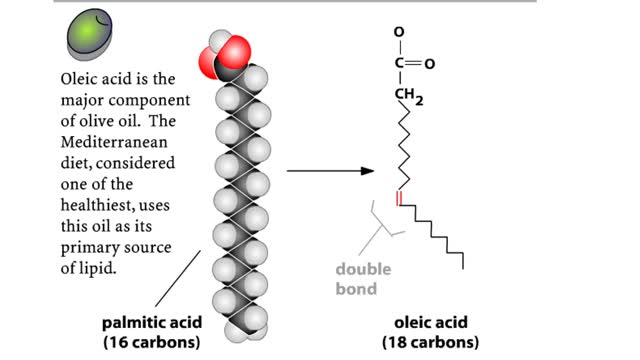
A triglyceride (also called triacylglycerol) is composed of three fatty acid molecules and one glycerol molecule. The fatty acids attach to the glycerol molecule by a covalent ester bond. The long hydrocarbon chain of each fatty acid makes the triglyceride molecule nonpolar and hydrophobic. Pa...
Lipid catabolism ( ketogenesis and oxidation of glycerol) and Lipid anabolism (lipogenesis)
By: HWC, Views: 12070
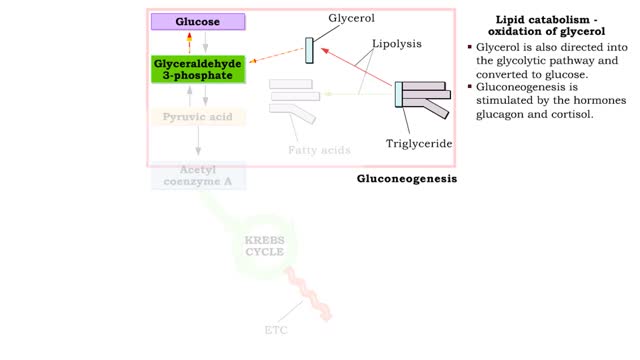
• During excessive beta oxidation, the two-carbon fatty acid fragments are converted into acidic ketone bodies. • Ketosis, the overproduction of ketone bodies, can lead to acidosis (ketoacidosis) of the blood. • After lipolysis, glycerol is converted to pyruvic acid. • Pyruvic aci...
Advertisement