Biological organic compounds
By: HWC
Date Uploaded: 05/05/2020
Tags: homeworkclinic.com Homework Clinic HWC Biological organic compounds covalent bonds nonpolar polar bond hogs iological macromolecules phosphate functional group carboxylic acid functional group amino functional group hydroxyl group hydrolysis reactions
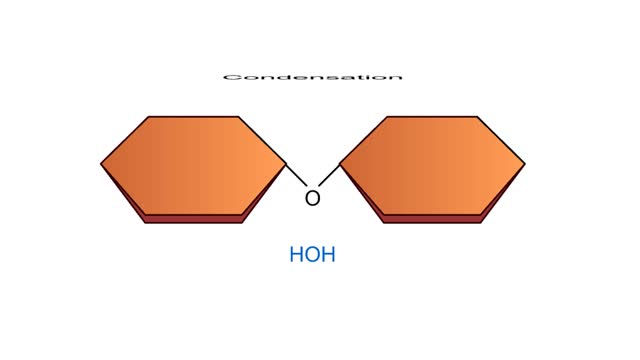
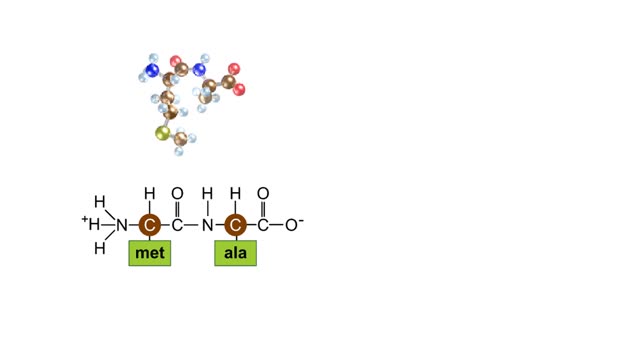
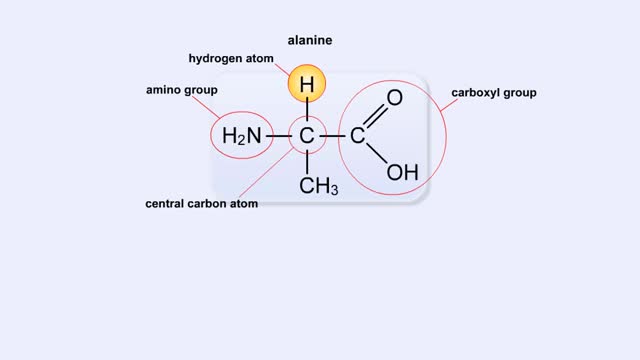
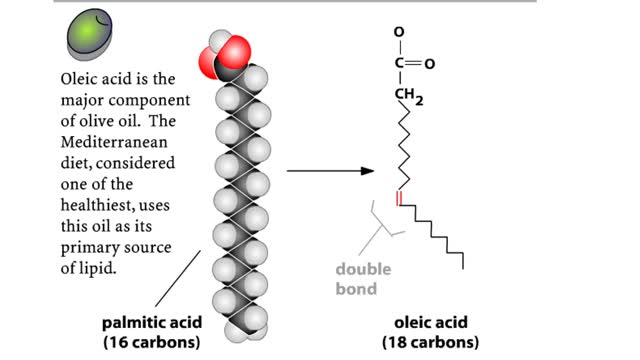
Biological organic compounds contain covalent bonds, mainly C-C and C-H bonds, but also both C and H bonded to such other atoms as O and N. Some of these covalent bonds are nonpolar. Others are polar, either because one atom in the group "hogs" electrons away from other atoms in the group, or because one or more atoms in the group can dissociate, so that the group becomes either negatively or positively charged. Here you see the principal functional groups found in biological macromolecules. The phosphate functional group is polar because it can lose either one or both H atoms, leaving the group with a negative charge: -HPO4: or -PO42. For this reason, molecules containing this group are soluble in water. Its ability to lose H+. makes this (Noun acidic). The carboxylic acid functional group is polar because the hydrogen can dissociate, meaning that the group loses an H+ ion and is left with a negative charge, -COO'. For this reason, molecules containing this group are soluble in water. Its ability to lose W makes this group acidic. The amino functional group is polar because the N can acquire an H+ ion, leaving the group with a positive charge: -NH3'. For this reason, molecules containing this group are soluble in water. Its ability to gain H+ makes this group basic. The hydroxyl group is polar because the O attracts electrons away from the H. Because of this polarity, compounds containing hydroxyl groups are usually soluble in water. This group participates in the synthetic dehydration and hydrolysis reactions involved in the formation and breakdown of biological macromolecules. C-H bonds are found in almost all biological molecules. Because these bonds are nonpolar. compounds containing only these bonds are not soluble in water.
Add To
You must login to add videos to your playlists.
Advertisement



Comments
0 Comments total
Sign In to post comments.
No comments have been posted for this video yet.