Search Results
Results for: 'fatty acid molecules'
Protein catabolism (Krebs cycle) and Protein anabolism (protein synthesis)
By: HWC, Views: 12314
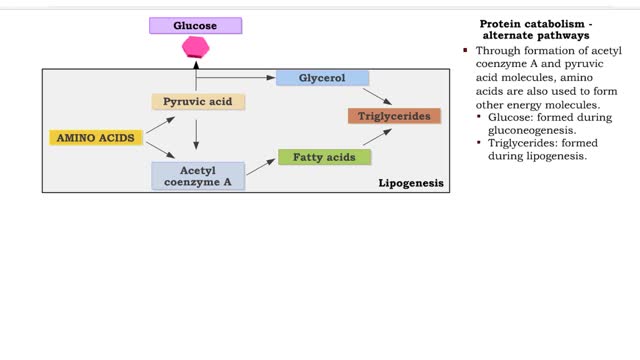
• Deaminated acids are brought into the Krebs cycle to be oxidized to CO2 and H2O. • Before entering the Krebs cycle, the deaminated acids are converted into intermediate products (pyruvic acid, acetyl coenzyme A, carbonic acids). • In the Krebs cycle, amino acids are oxidized to form r...
Digestive chemicals - water, gastric acid, bile & bicarbonate
By: HWC, Views: 11465
• Water is the most abundant molecule in ingested fluids. • Water plays a primary role in hydrolytic digestive reactions. • Helps liquefy and transport digestive foodstuffs down the tract. • Transports secretions from accessory digestive organs to gastrointestinal tract. • Aids ...
Lipid digestion - mouth, stomach and small intestine
By: HWC, Views: 11868
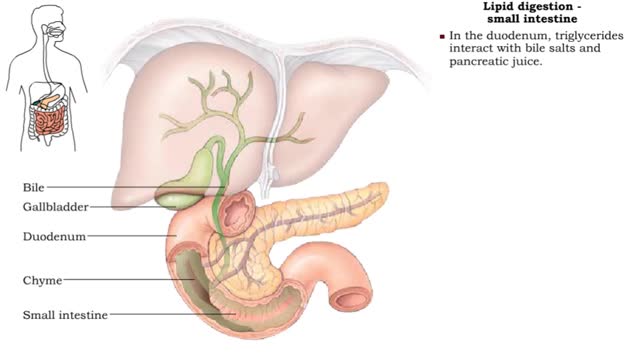
• Lipid digestion takes place primarily in the small intestine; some occurs in the mouth and stomach. • Lipases are enzymes that break down triglycerides and phospholipids. • Lingual and gastric lipases hydrolyze a small amount of triglycerides. • End products are fatty acids and...
Non-polar compounds - insolubility
By: HWC, Views: 11799
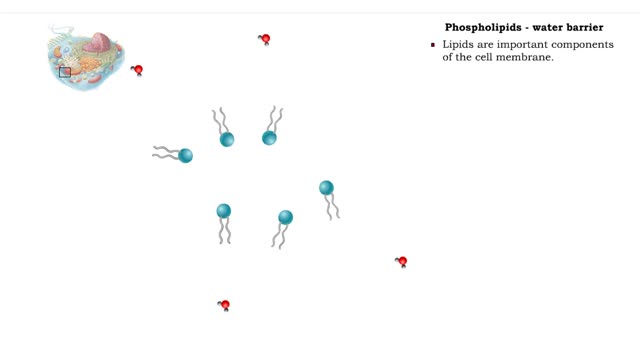
• A non-polar molecule has uniform distribution of electrons. • Non-polar compounds like fatty acids in lipids have a high proportion of carbon and hydrogen. • Lipids possess no charge or partial charge. • Lipids are not attracted to water molecules. • Lipids are not soluble in...
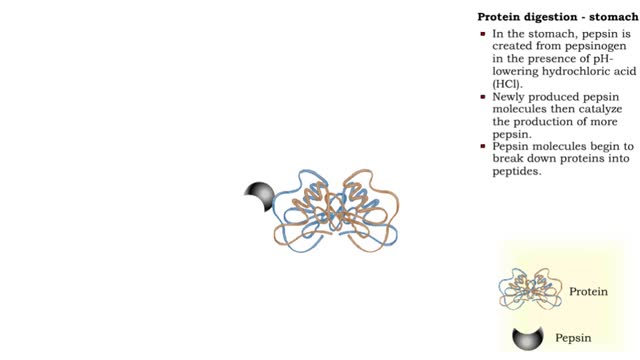
Protein digestion - stomach & small intestine
By: HWC, Views: 11257
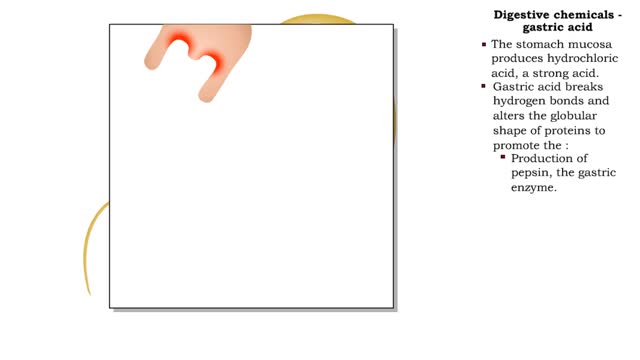
• Protein digestion occurs in the stomach and small intestine. • The stomach enzyme pepsin initiates the process. • Pancreatic and intestinal brush border enzymes complete the digestive process. • In the stomach, pepsin is created from pepsinogen in the presence of pH-lowering hyd...
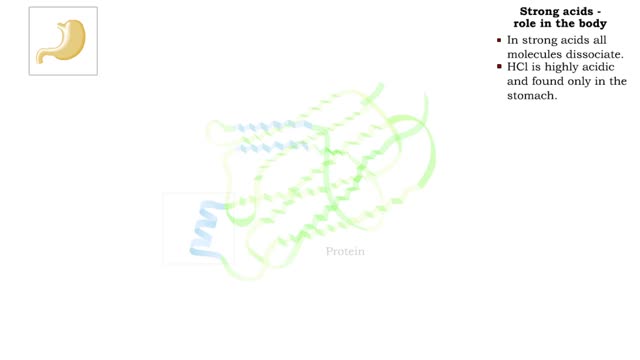
The pH scale - Strong acids and Weak acids
By: HWC, Views: 11840
The pH scale • Expresses concentration of H+. • range: 0-14. • 7 is neutral. • Less 7 is acid. • greater 7 is basic (alkaline). Strong acids - role in the body ■ In strong acids all molecules dissociate. ■ HC1 is highly acidic and found only in the stomach. • H...
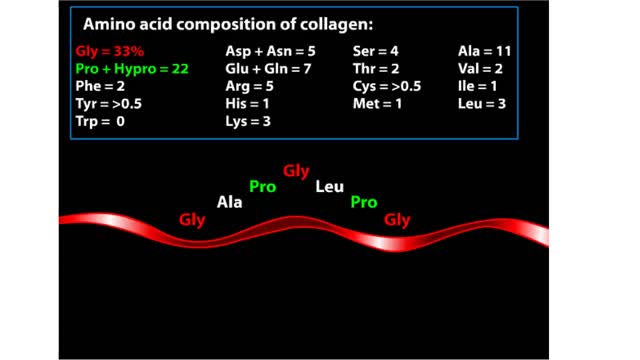
Major Elements in Biological Molecules: Proteins
By: HWC, Views: 11198
Proteins are chains of amino acids linked by peptide bonds. The 20 different amino acids used to make all proteins differ only in their side chains, and the properties of these side chains account for the great diversity of protein structure and function. Collagen is an example of how a prote...
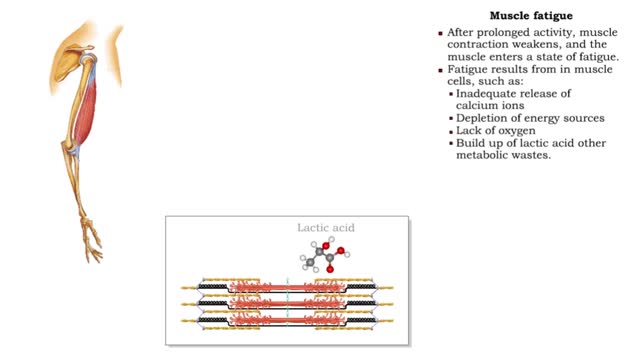
By: HWC, Views: 11438
• After prolonged activity, muscle contraction weakens, and the muscle enters a state of fatigue. • Fatigue results from in muscle cells, such as: • Inadequate release of calcium ions • Depletion of energy sources • Lack of oxygen • Build up of lactic acid other metabolic w...
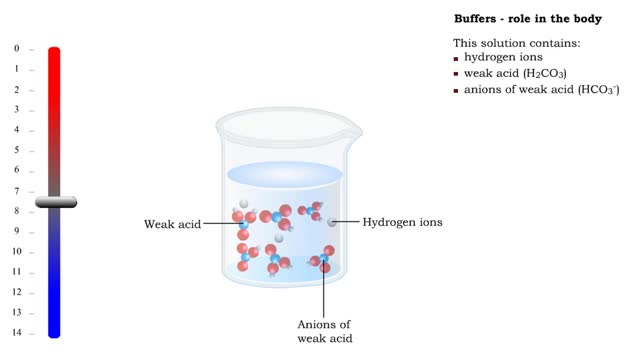
Buffers definition and the role of buffer in the body
By: HWC, Views: 11743
■ Too many H+ break hydrogen bonds and a protein comes apart. ■ Buffers react with excess H+ to protect proteins from breaking down. ■ Buffers consist of weak acid plus anions of that weak acid. This solution contains: • hydrogen ions • weak acid (H2CO3) • anions of we...
Advertisement