Search Results
Results for: 'hydrogen bonds'
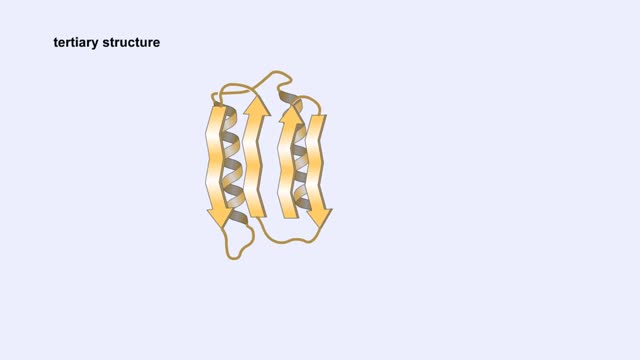
Protein Structure - Primary, Secondary, Tertiary and Quaternary
By: HWC, Views: 11589
A protein's first order structure, or primary structure, begins with the amino acid sequence of the polypeptide chain. The 20 different amino acids can be arranged in an infinite number of sequences. For example, the hormone insulin, which regulates the uptake of glucose from the blood into ce...
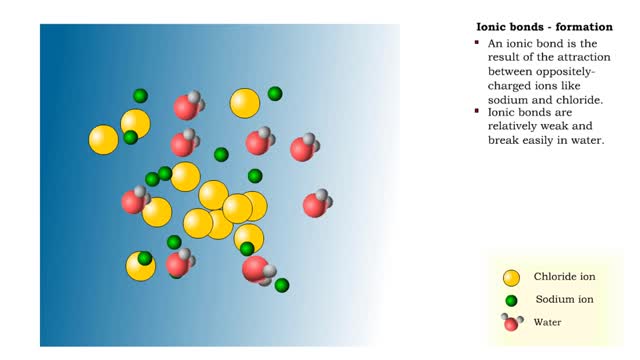
Ionic bonds - role of ions in the body
By: HWC, Views: 11989
Ions • Atoms fill up the outer orbital by transferring electrons from one atom to another. • Atoms now bear a charge and are called ions. • Sodium ion, losing an electron, has a +1 charge. • Chlorine ion, gaining an electron, has a -1 charge. Formation • An ionic bond is t...
By: HWC, Views: 11333
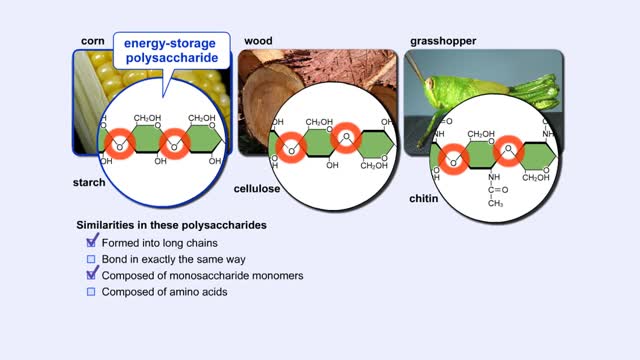
More complex sugars are called polysaccharides (from "poly" meaning "many" and "saccharum" meaning "sugar"). Many things in nature are made of polysaccharides. Here we show one of the polysaccharides in corn, another in wood, and another in the exoskeletons of insects like grasshoppers. How are a...
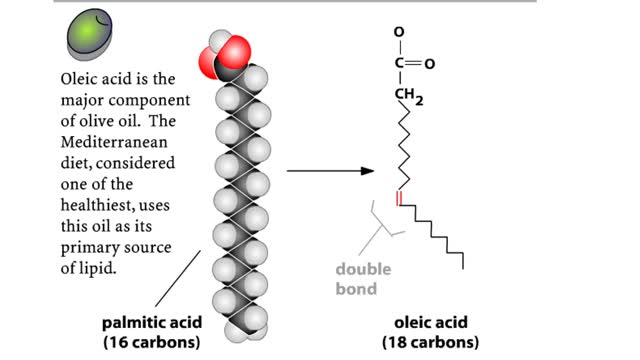
Major Elements in Biological Molecules: Lipids
By: HWC, Views: 11113
A triglyceride (also called triacylglycerol) is composed of three fatty acid molecules and one glycerol molecule. The fatty acids attach to the glycerol molecule by a covalent ester bond. The long hydrocarbon chain of each fatty acid makes the triglyceride molecule nonpolar and hydrophobic. Pa...
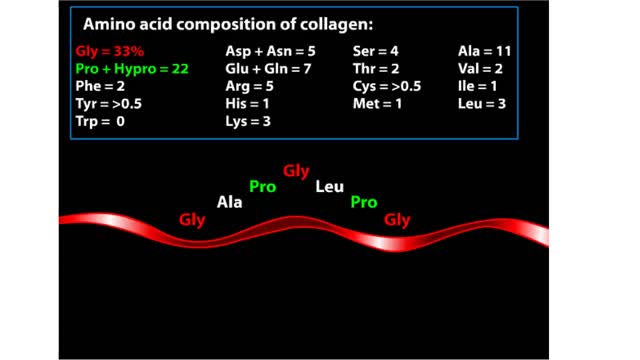
Major Elements in Biological Molecules: Proteins
By: HWC, Views: 11218
Proteins are chains of amino acids linked by peptide bonds. The 20 different amino acids used to make all proteins differ only in their side chains, and the properties of these side chains account for the great diversity of protein structure and function. Collagen is an example of how a prote...
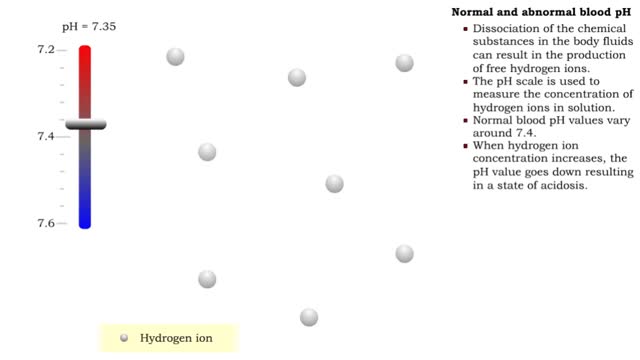
By: HWC, Views: 11811
• Dissociation of the chemical substances in the body fluids can result in the production of free hydrogen ions. • The pH scale is used to measure the concentration of hydrogen ions in solution. • Normal blood pH values vary around 7.4. • When hydrogen ion concentration increases, t...
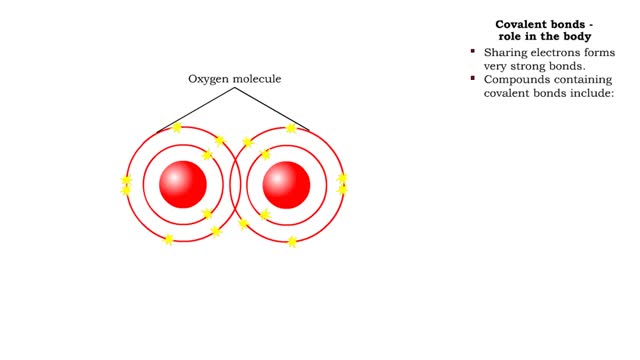
Covalent bonds - role in the body
By: HWC, Views: 11684
A covalent bond is formed when atoms share one or more pairs of electrons. This is opposed to an ionic bond, where electrons are actually transferred from one atom to another. Formation • Atoms fill up the outer orbital by sharing electrons. • Two oxygen atoms sharing electrons form on...
Anatomy and Chemical Makeup of a Single Hair (Animation)
By: HWC, Views: 10105
The hair's outer cuticle surrounds hair cells filled with tough keratin macrofibrils. Each macrofibril consists of smaller microfibrils. A microfibril is made up of three keratin polypeptide chains. The chains are linked together by disulfide bonds. A hair consists of keratin chains held...
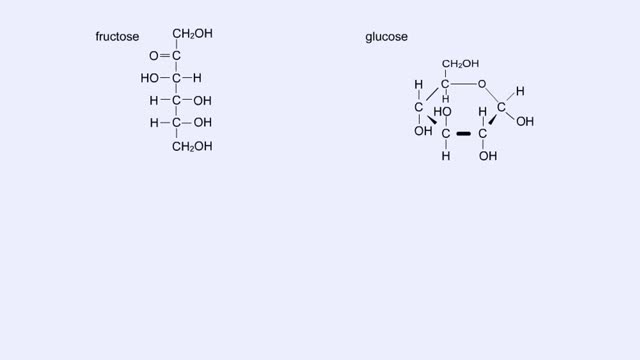
By: HWC, Views: 11479
Here are the molecular structures of three simple sugars: glucose, ribose, and fructose. Look at these simple sugars and identify what characteristics they all share. As you can see, all of the carbohydrates have carbon, hydrogen, and oxygen in a ratio of 1:2:1 and there is always a double bo...
Advertisement