Simple and Double Sugar
By: HWC
Date Uploaded: 03/01/2020
Tags: homeworkclinic.com Homework Clinic HWC Double Sugar simple sugar glucose ribose fructose carbohydrates Straight Chain double bond monosaccharides Disaccharides maltose
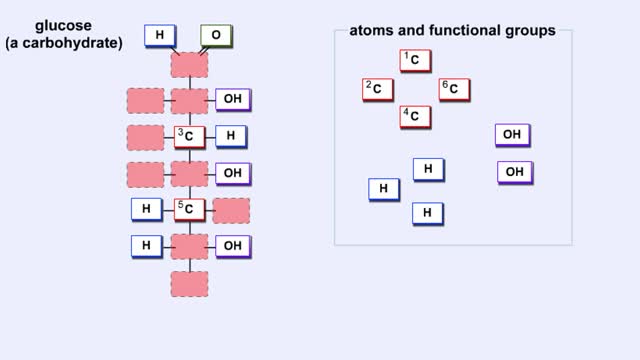
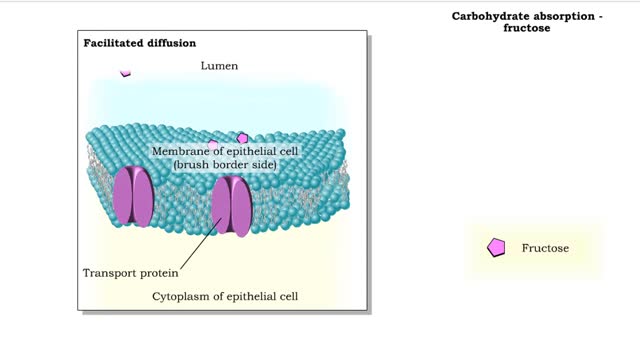
Here are the molecular structures of three simple sugars: glucose, ribose, and fructose. Look at these simple sugars and identify what characteristics they all share. As you can see, all of the carbohydrates have carbon, hydrogen, and oxygen in a ratio of 1:2:1 and there is always a double bond between a carbon atom and an oxygen atom. Carbon forms the backbone of the molecule. These are the basic characteristics of all carbohydrates. Another fact about carbohydrates is that they can appear in two forms. They can be in the form of a straight chain, like the examples here. Or, they can take the form of a ring. Because this ring structure is so important, let's take a closer look. Representations like you see here of atoms and bonds are used to show you these arrangements as they exist in nature. This one is called a "Straight Chain" model, which shows the symbol of each atom and a straight line that represents a covalent bond. A double (parallel) line represents a double covalent bond. However, carbohydrates usually exist as rings in solution. To produce these rings, the carbon atom that is double-bonded to oxygen bonds to the oxygen atom in one of the OH groups. For example, glucose forms a ring consisting of five carbon atoms and one oxygen atom. Remember that glucose forms a ring consisting of five carbon atoms and one oxygen atom, and that the carbon atom that is double-bonded to oxygen bonds to the oxygen atom in one of the OH groups. See how a ring is formed with a bond between the carbon atom and the oxygen atom? Now try to form a ring for fructose. Fructose forms a ring consisting of four carbon atoms and one oxygen atom. As we have seen, monosaccharides are a single chain or ring of a carbohydrate. For instance, glucose and fructose are monosaccharides. Disaccharides (from "di" meaning "two" and "saccharum" meaning "sugar") are formed by the joining together of two monosaccharide molecules. Disaccharides are "double sugars" because two single sugars have been linked together to form this type of molecule. We obtain double sugars from several different food groups. Look at the table provided. Notice that all three disaccharides have glucose as one of their building blocks. From the pool of monosaccharides, identify the second building block for maltose, sucrose, and lactose. As you can see both halves of the disaccharides match the two monosaccharides. There are many other monosaccharides that can combine in different ways to form numerous disaccharides. But, let's move on to see how even more complex carbohydrates can be formed.
Add To
You must login to add videos to your playlists.
Advertisement




Comments
0 Comments total
Sign In to post comments.
No comments have been posted for this video yet.