Search Results
Results for: 'calcium ions'
Buffers definition and the role of buffer in the body
By: HWC, Views: 10912
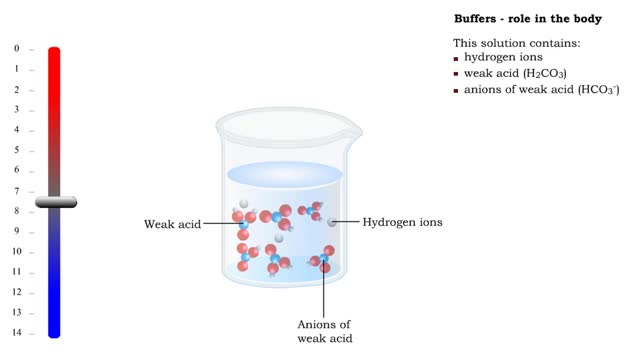
■ Too many H+ break hydrogen bonds and a protein comes apart. ■ Buffers react with excess H+ to protect proteins from breaking down. ■ Buffers consist of weak acid plus anions of that weak acid. This solution contains: • hydrogen ions • weak acid (H2CO3) • anions of we...
Protein catabolism - deamination
By: HWC, Views: 11071
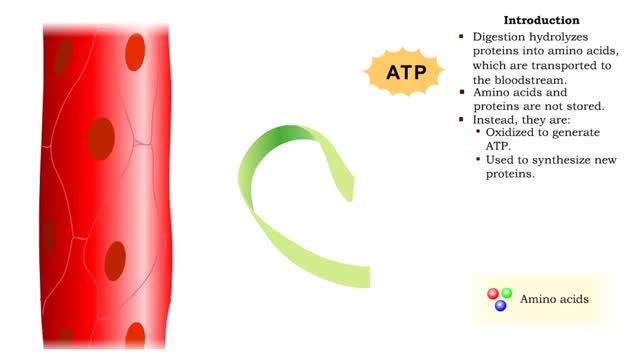
• Digestion hydrolyzes proteins into amino acids, which are transported to the bloodstream. • Amino acids and proteins are not stored. • Instead, they are: • Oxidized to generate ATP. • Used to synthesize new proteins. • Converted to carbohydrates or lipids for storage (if e...
Atrial natriuretic peptide (vasodilation) & Aldosterone
By: HWC, Views: 10609
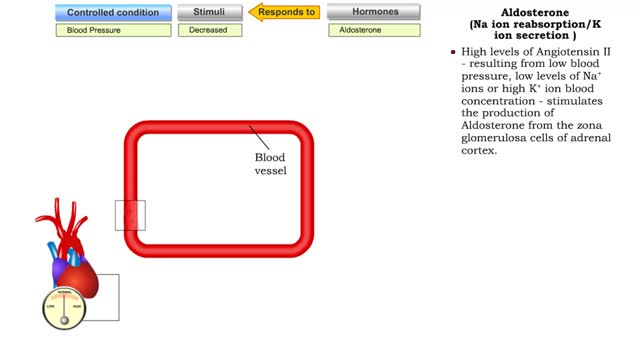
• Certain situations will cause the body's stress level to rise. • increased blood pressure will stretch the atria of the heart, stimulating the secretion of atria natriuretic peptide (MP). • ANP causes muscle cells in blood vessels to relax. • Blood pressure is lowered as a result ...
Role of the respiratory system - effect of altered ventilation rates
By: HWC, Views: 11482
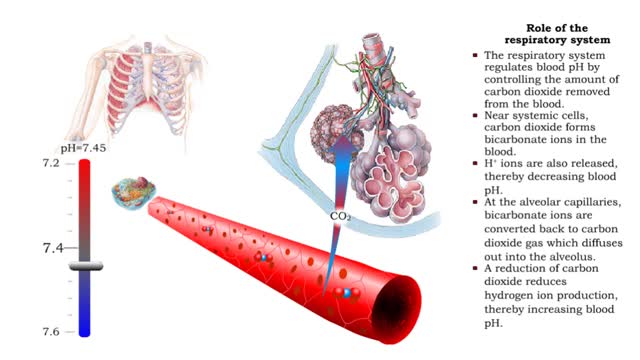
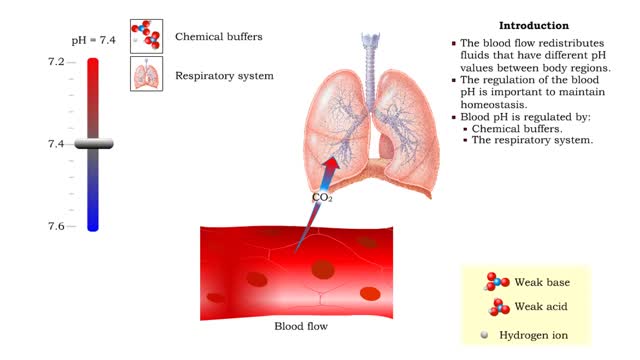
• The respiratory system regulates blood pH by controlling the amount of carbon dioxide removed from the blood. • Near systemic cells, carbon dioxide forms bicarbonate ions in the blood. H+ ions are also released, thereby decreasing blood pH. • At the alveolar capillaries, bicarbonate io...
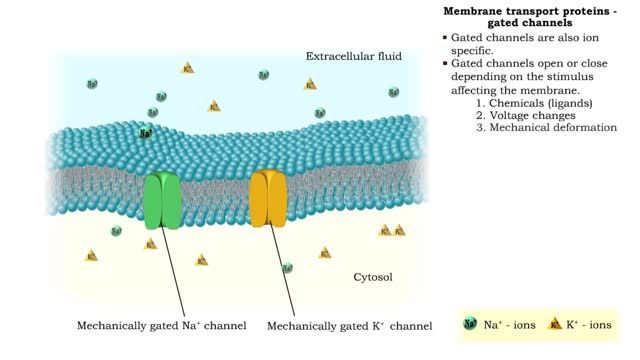
Membrane transport proteins - pores, gated channels and pumps
By: HWC, Views: 11038
• a Three different types of membrane ion transport proteins are required to produce and carry electrical signals: • Pores • Gated channels • Na+/ K+ pump • Pores are always open and allow the diffusion of Na+ and K+ ions across the membrane, down their concentration gradients...
Role of the respiratory system - effect of altered ventilation rates
By: HWC, Views: 10752
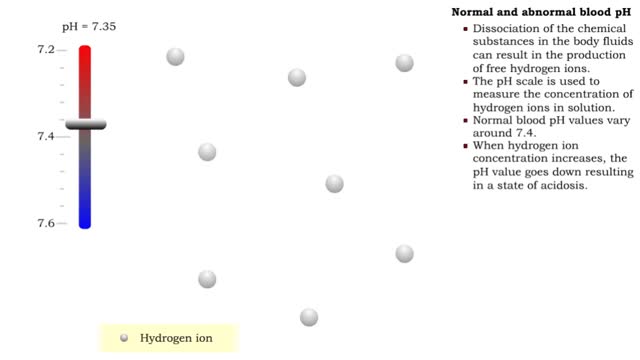
• Dissociation of the chemical substances in the body fluids can result in the production of free hydrogen ions. • The pH scale is used to measure the concentration of hydrogen ions in solution. • Normal blood pH values vary around 7.4. • When hydrogen ion concentration increases, t...
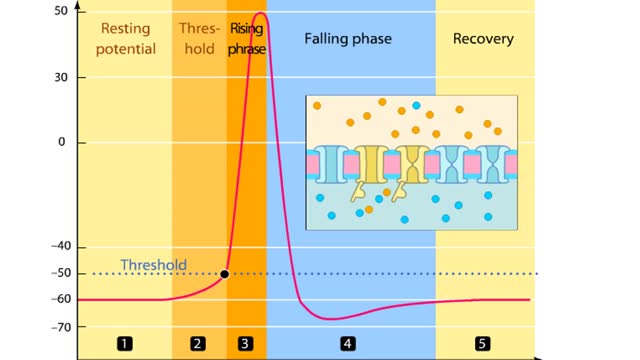
Phases of an Action Potential - Resting Potential, Threshold, Rising, Falling, & Recovery Phases
By: HWC, Views: 10293
In this tutorial, we will review the phases of an action potential measured from a small area of a neuron's membrane. The action potential can be divided into five phases: the resting potential, threshold, the rising phase, the falling phase, and the recovery phase. When the neuron is at rest,...
By: HWC, Views: 10765
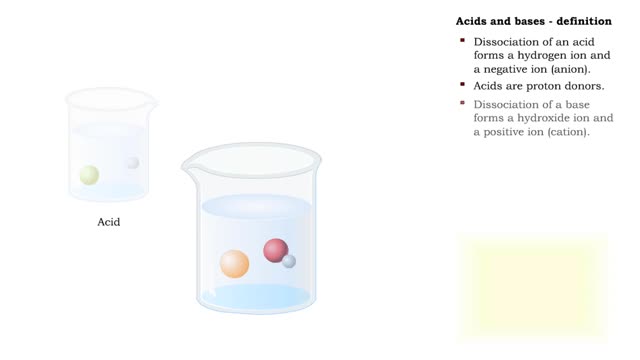
Acids and bases are found all around your house. For example, if you open your pantry or refrigerator, you might see a lot of acids. Fruit juice, soda pop, vinegar, and milk are all examples of acids. The word acid actually comes from a Latin term meaning ''sour.'' Many materials, like sugar for ...
By: HWC, Views: 10965
• Dissociation of the chemical substances in the body fluids can result in the production of free hydrogen ions. • The pH scale is used to measure the concentration of hydrogen ions in solution. • Normal blood pH values vary around 7.4. • When hydrogen ion concentration increases, t...
Advertisement