Search Results
Results for: 'lipid bilayer'
Acid-base imbalances - respiratory acidosis and alkalosis
By: HWC, Views: 12052
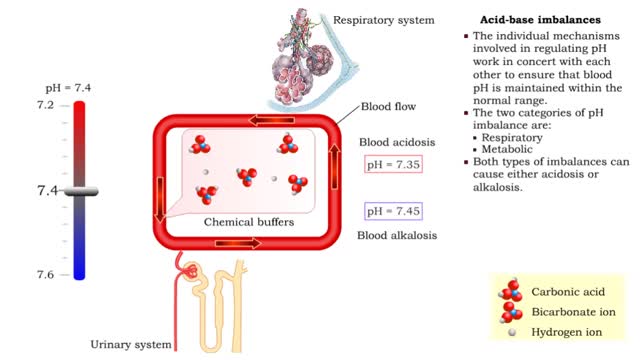
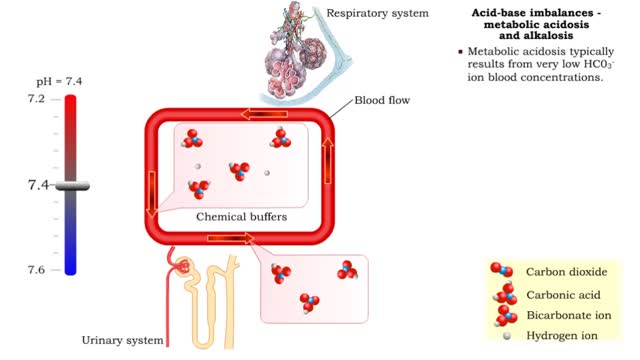
• The individual mechanisms involved in regulating pH work in concert with each other to ensure that blood pH is maintained within the normal range. • The two categories of pH imbalance are: • Respiratory • Metabolic • Both types of imbalances can cause either acidosis or alka...
By: HWC, Views: 12294
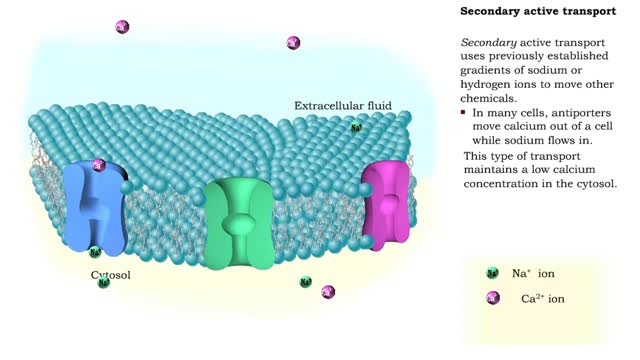
Energy stored (in a hydrogen or sodium concentration gradient) is used to drive other substances against their own concentration gradients Secondary active transport, is transport of molecules across the cell membrane utilizing energy in other forms than ATP. In many cells, antiporters mov...
Non-polar compounds - insolubility
By: HWC, Views: 11812
• A non-polar molecule has uniform distribution of electrons. • Non-polar compounds like fatty acids in lipids have a high proportion of carbon and hydrogen. • Lipids possess no charge or partial charge. • Lipids are not attracted to water molecules. • Lipids are not soluble in...
By: HWC, Views: 12073
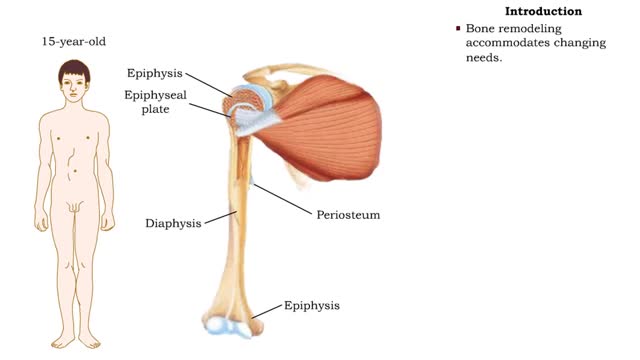
• After birth, bones grow in thickness and length. • Bones grow in diameter via appositional growth at the periosteum. • Epiphyseal plates enable lengthwise growth of long bones, such as the humerus, by interstitial growth. • Bone remodeling accommodates changing needs. • While th...
By: HWC, Views: 11809
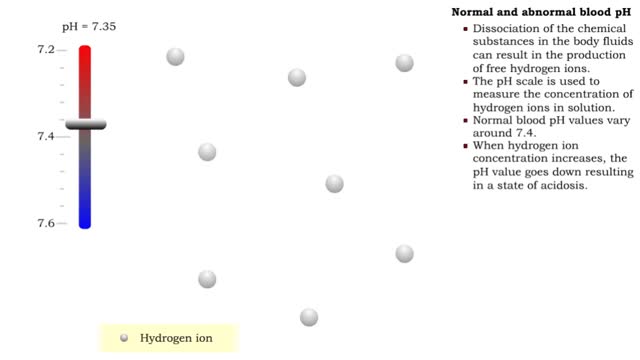
• Dissociation of the chemical substances in the body fluids can result in the production of free hydrogen ions. • The pH scale is used to measure the concentration of hydrogen ions in solution. • Normal blood pH values vary around 7.4. • When hydrogen ion concentration increases, t...
Simple Diffusion - Ion transport
By: HWC, Views: 11733
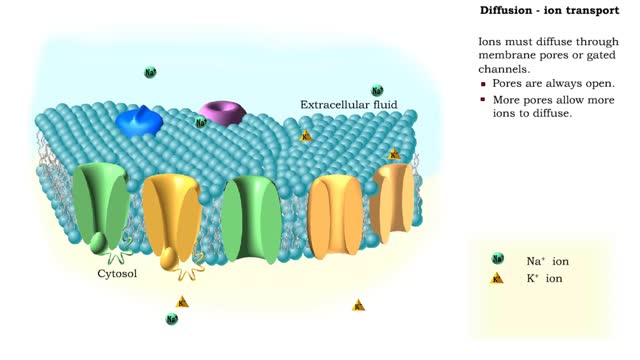
In the process of diffusion, a substance tends to move from an area of high concentration to an area of low concentration until its concentration becomes equal throughout a space. Ions must diffuse through membrane pores or gated channels. Pores are always open. More pores allow more ions...
Acid-base imbalances - compensation of metabolic acidosis and alkalosis
By: HWC, Views: 11859
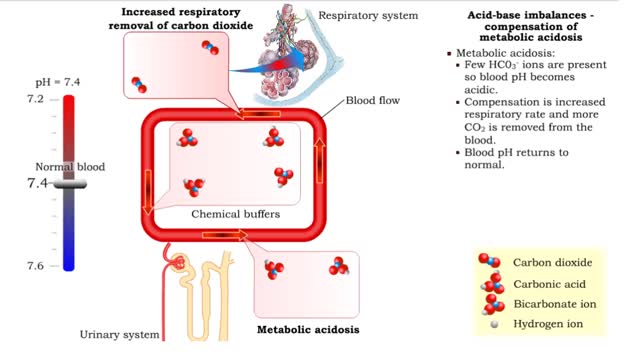
1. Metabolic acidosis: • Few HC03- ions are present so blood pH becomes acidic. • Compensation is increased respiratory rate and more CO2 is removed from the blood. • Blood pH returns to normal. 2. Metabolic alkalosis: • Many HC03- ions are present so blood pH becomes alkaline...
Mechanisms of capillary exchange (transcytosis & bulk flow)
By: HWC, Views: 11393
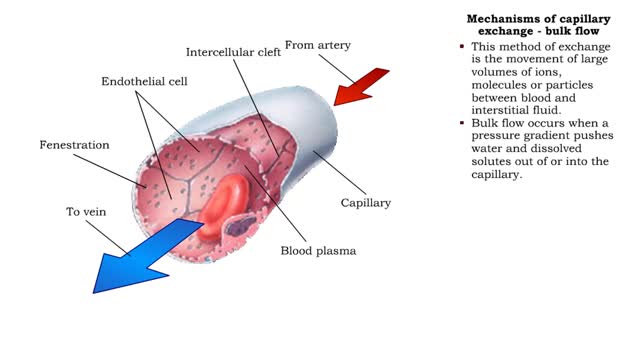
■ This method of capillary exchange is mainly used to transport small amounts of large, lipid-insoluble (water soluble) molecules, such as large proteins. ■ Substances, packaged in vesicles, move through endothelial cells via endocytosis and exocytosis. ■ This method of exchange is th...
Acid-base imbalances - compensation of respiratory acidosis and alkalosis
By: HWC, Views: 11959
• When one pH balancing system is affected then the other balancing system attempts to correct, or compensate for, the pH imbalance. - Respiratory acidosis: • Excessive CO2 is present so blood pH becomes acidic. • Compensation is increased secretion of H+ into urine and reabsorption ...
Advertisement