Search Results
Results for: 'Chemical digestion'
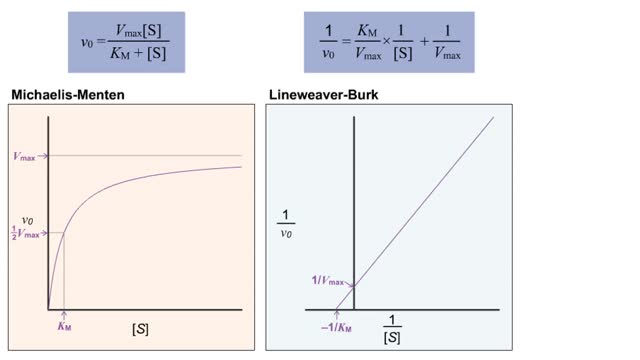
Virtual Enzyme Kinetics & Lineweaver Burk Plot
By: HWC, Views: 11155
• The double-reciprocal (also known as the Lineweaver-Burk) plot is created by plotting the inverse initial velocity (1/V0) as a function of the inverse of the substrate concentration (1/[S]). • This plot is a useful way to determined different inhibitors such as competitive, uncompetitive...
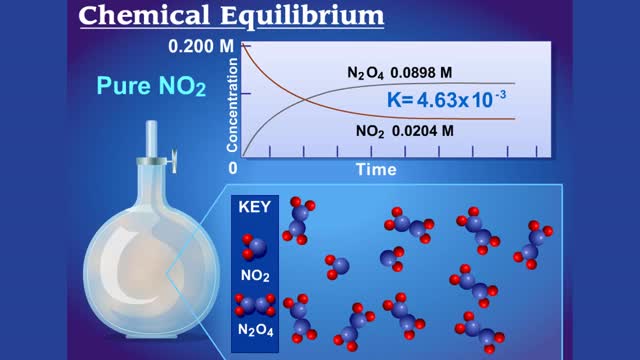
Chemical Equilibrium between N2O4 (colorless gas) and NO2 (brown gas)
By: HWC, Views: 10863
For a system at equilibrium: ◆ both forward and reverse reactions are occurring simultaneously ◆ rate of forward reaction must equal rate of reverse reaction OR Rate forward = Rate reverse ◆ concentrations of reactants and products remain constant with time the equilibrium positio...
Neurotransmission at chemical synapses & Excitory and inhibitory potentials
By: HWC, Views: 11581
• A series of events occur at chemical synapses in order to communicate with the adjacent cell. • The action potential arrives at the presynaptic membrane. • The depolarization phase of the action potential opens voltage gated Ca+ channels. • increased inflow of Ca+' into the cyto...
By: Administrator, Views: 14584
In the nervous system, a synapse is a structure that permits a neuron (or nerve cell) to pass an electrical or chemical signal to another neuron or to the target effector cell. Synapses are essential to neuronal function: neurons are cells that are specialized to pass signals to individual tar...
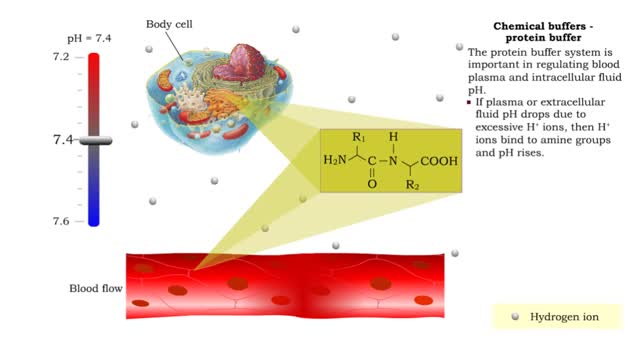
Chemical Buffers - protein buffer, phosphate buffer system and bicarbonate buffer system
By: HWC, Views: 11804
• There are a variety of chemicals in body fluids that prevent the fluids from undergoing large changes in. • These chemicals buffer or regulate fluctuations in H+ concentration. • Chemical buffers: • Bind to H+ ions when there are too many in a solution so pH remains normal. •...
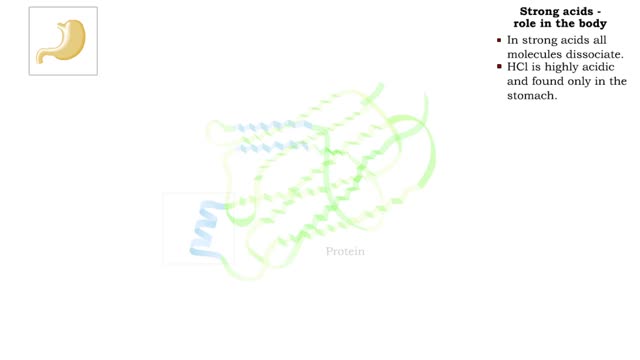
The pH scale - Strong acids and Weak acids
By: HWC, Views: 11718
The pH scale • Expresses concentration of H+. • range: 0-14. • 7 is neutral. • Less 7 is acid. • greater 7 is basic (alkaline). Strong acids - role in the body ■ In strong acids all molecules dissociate. ■ HC1 is highly acidic and found only in the stomach. • H...
By: HWC, Views: 10781
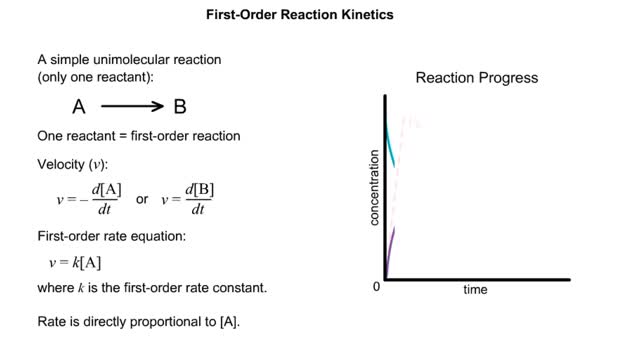
✔ https://HomeworkClinic.com ✔ https://Videos.HomeworkClinic.com ✔ Ask questions here: https://HomeworkClinic.com/Ask Follow us: ▶ Facebook: https://www.facebook.com/HomeworkClinic ▶ Review Us: https://trustpilot.com/review/homeworkclinic.com Kinetics is the study of the rat...
Cellular slime mold life cycle Animation
By: HWC, Views: 5933
Life cycle of Dictyostelium discoideum, a cellular slime mold Animation. Amoeba-like slime mold cells live in the soil, where they feed on bacteria. The free-living cells grow and reproduce by mitosis. When food dwindles, the amoebas stream toward one another in response to a chemical...
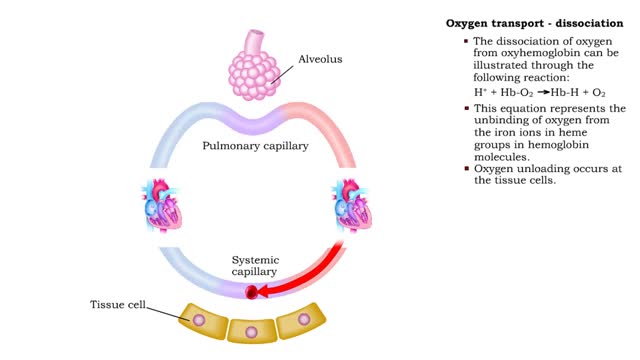
Oxygen transport: association and dissociation & Factors that affect hemoglobin's saturation with O2
By: HWC, Views: 11483
• The production of oxyhemoglobin can be illustrated through the following reaction: 02 + Hb-H --) Hb-02 + H+ • This equation represents the binding of oxygen to the iron ions in heme groups in hemoglobin molecules. • Oxygen binding or loading occurs at the lungs • The dissociatio...
Advertisement