Search Results
Results for: 'hydrogen'
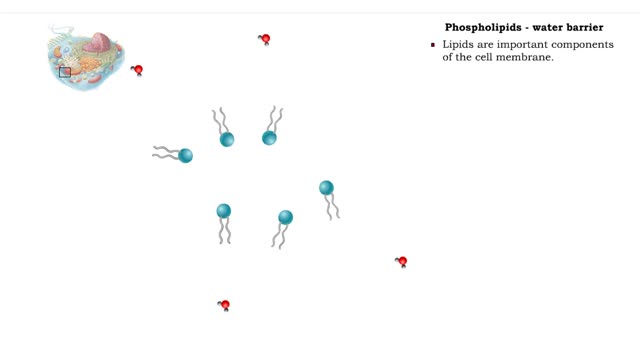
Non-polar compounds - insolubility
By: HWC, Views: 11421
• A non-polar molecule has uniform distribution of electrons. • Non-polar compounds like fatty acids in lipids have a high proportion of carbon and hydrogen. • Lipids possess no charge or partial charge. • Lipids are not attracted to water molecules. • Lipids are not soluble in...
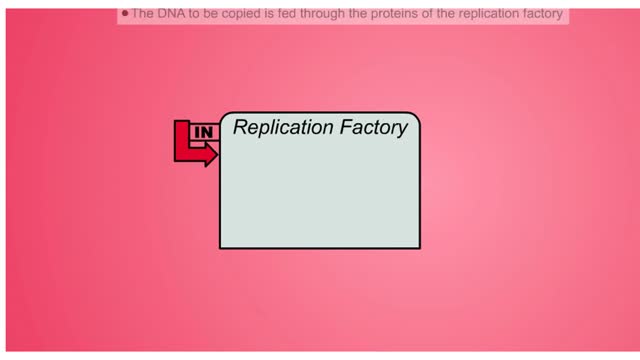
DNA Replication Factory and Protein
By: HWC, Views: 10944
DNA (deoxyribose nucleic acid) carries all the genetic information needed to re-create itself and to pass on the characteristics of the organism. The “factory” model of DNA replication hypothesizes a specific nuclear structure in which the molecular machinery for replication forks are brou...
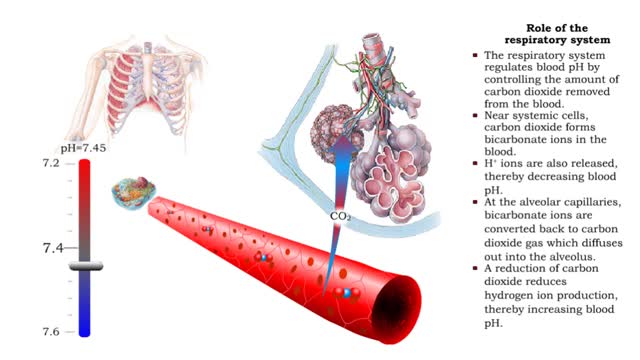
Role of the respiratory system - effect of altered ventilation rates
By: HWC, Views: 11935
• The respiratory system regulates blood pH by controlling the amount of carbon dioxide removed from the blood. • Near systemic cells, carbon dioxide forms bicarbonate ions in the blood. H+ ions are also released, thereby decreasing blood pH. • At the alveolar capillaries, bicarbonate io...
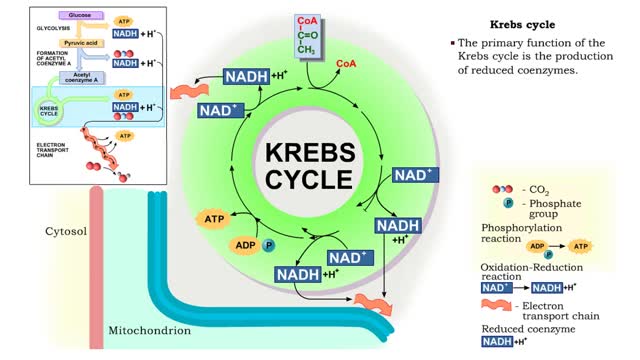
Krebs cycle : Formation of acetyl coenzyme A and Electron transport chain
By: HWC, Views: 11559
The oxidation of glucose to produce ATP is cellular respiration. Four sets of reactions are involved: Glycolysis Formation of acetyl coenzyme A Krebs cycle reactions Electron transport chain reactions • The second pathway of glucose catabolism, formation of acetyl coenzyme A, is a transi...
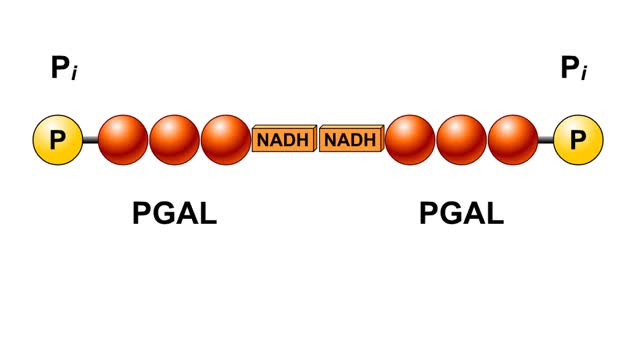
By: HWC, Views: 5321
In glycolysis, a six-carbon glucose molecule is split into two three-carbon pyruvate molecules. In this animation, each carbon molecule is represented by a red ball. The end products of glycolysis are two molecules of pyruvate. Glycolysis is the breakdown of glucose into two molecules of ...
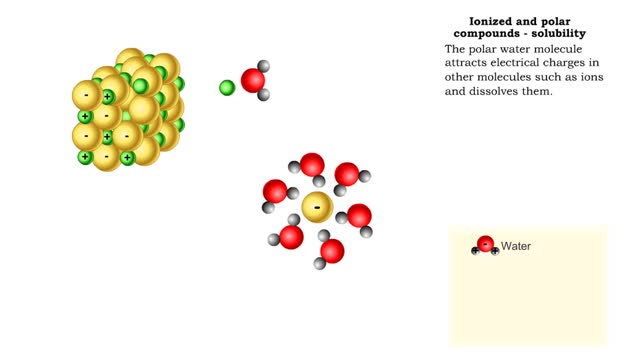
Properties of water -structure of water and polarity (Ionized and polar compounds)
By: HWC, Views: 11482
■ Water transports most of the molecules in the body. ■ The structure of a water molecule allows it to dissolve other molecules. ■ Shared electrons spend more time near the oxygen atom. ■ Oxygen end has a partial negative charge. ■ Hydrogen ends have a partial positive charge....
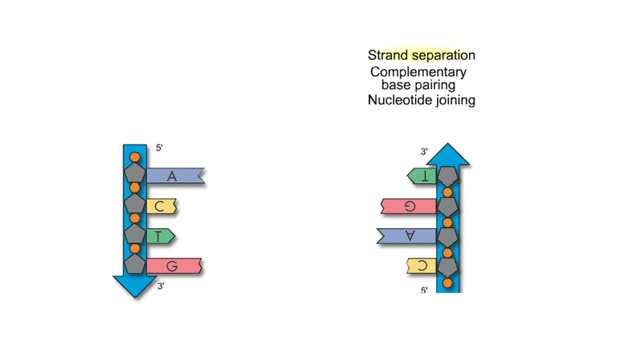
Subunits of DNA And Semi Conservative Replication
By: HWC, Views: 7639
Adenine is a purine with a double-ring structure. In double-stranded DNA, adenine base-pairs with thymine. Guanine is a purine with a double-ring structure. In double-stranded DNA, guanine base-pairs with cytosine. Thymine is a pyrimidine with a single-ring structure. In double-stranded DNA, th...
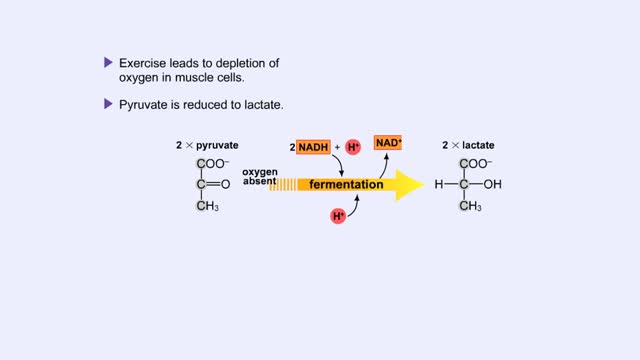
Fermentation - When Oxygen Is Absent, Pyruvate to Lactate & Pyruvate to Ethanol
By: HWC, Views: 10815
Pyruvate is the end product of glycolysis. If oxygen is present, pyruvate enters the mitochondrion where further energy yielding reactions of the Krebs cycle will take place. However, if oxygen is not present, pyruvate will enter a pathway called fermentation. This pathway regenerates NAD+ fro...
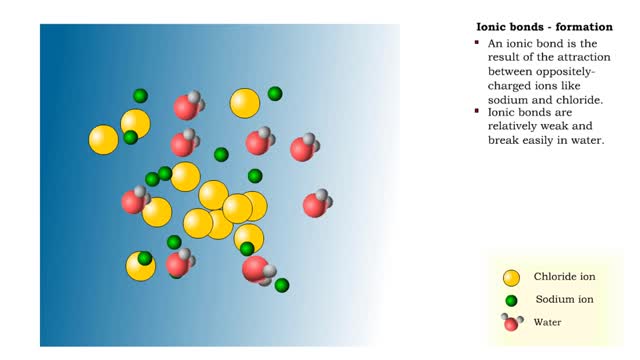
Ionic bonds - role of ions in the body
By: HWC, Views: 11628
Ions • Atoms fill up the outer orbital by transferring electrons from one atom to another. • Atoms now bear a charge and are called ions. • Sodium ion, losing an electron, has a +1 charge. • Chlorine ion, gaining an electron, has a -1 charge. Formation • An ionic bond is t...
Advertisement