Search Results
Results for: 'Structure of Amino Acid'
By: HWC, Views: 10908
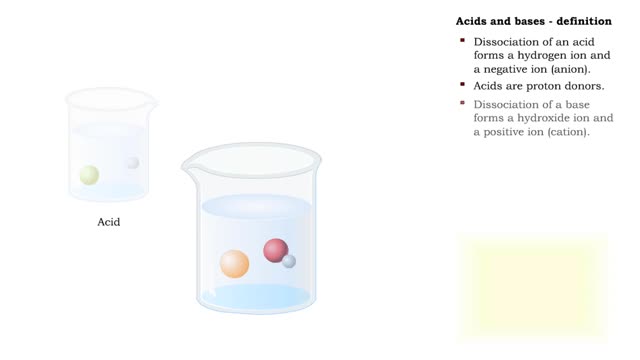
Acids and bases are found all around your house. For example, if you open your pantry or refrigerator, you might see a lot of acids. Fruit juice, soda pop, vinegar, and milk are all examples of acids. The word acid actually comes from a Latin term meaning ''sour.'' Many materials, like sugar for ...
By: HWC, Views: 10204
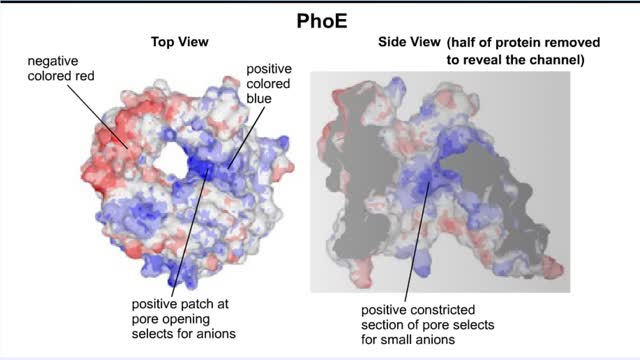
Transmembrane channels, also called membrane channels, are pores within a lipid bilayer. The channels can be formed by protein complexes that run across the membrane or by peptides. They may cross the cell membrane, connecting the cytosol, or cytoplasm, to the extracellular matrix. Membrane po...
Nucleic acid digestion - brush border enzymes, end products & transport mechanism
By: HWC, Views: 10869
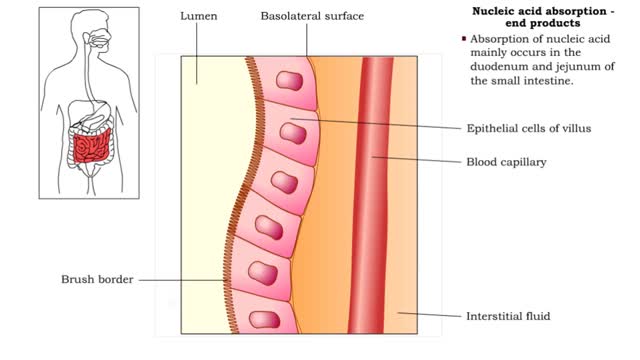
• Further digestion occurs at the microvilli (brush border) of the epithelial cells of the villi in the small intestine. • Two brush border enzymes complete nucleic acid digestion: • Phosphatases, which catalyze the cleavage of a phosphate to form a nucleoside (nitrogenous base and pent...
Bond types - Atomic structure and basis of bonds
By: HWC, Views: 11444
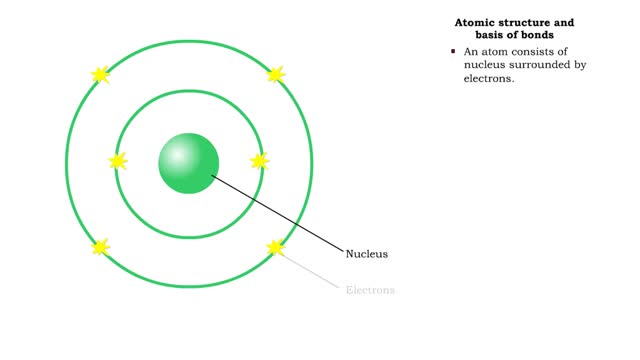
• Chemical bonds are fundamental to the structure and function of many types of molecules, such as proteins, carbohydrates, lipids, nucleic acids, gases, salts and water. ■ These molecules are composed of atoms that are held together by three different types of bonds. • The three types ...
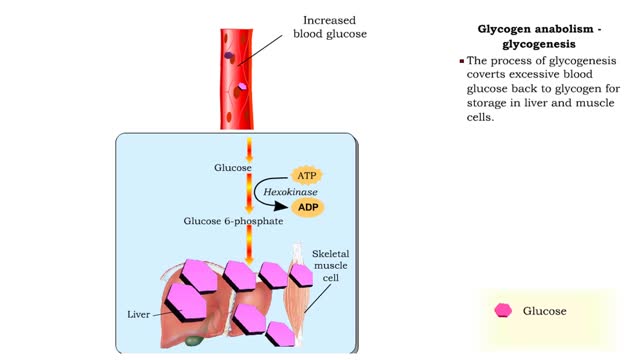
Glucose anabolism reactions: Glycogenolysis and Gluconeogenesis
By: HWC, Views: 11400
• Glucose not needed immediately is stored as glycogen. The process that creates it is glycogenesis. • When ATP is needed for body activities, stored glycogen is broken down by a process called glycogenolysis. • Glucose can be formed through two different anabolic reactions: • Glycog...
By: HWC, Views: 10684
The life cycle of a typical protein begins with its synthesis on a ribosome. As the polypeptide chain grows, molecules of a chaperone protein bind along its length. This prevents misfolding of the nascent polypeptide. ATP binding causes chaperone release. For most proteins, the polypeptide th...
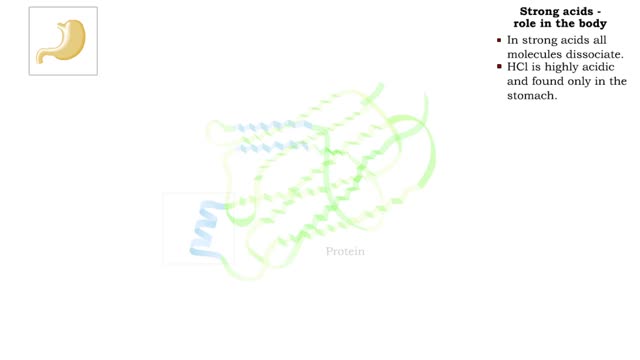
The pH scale - Strong acids and Weak acids
By: HWC, Views: 11111
The pH scale • Expresses concentration of H+. • range: 0-14. • 7 is neutral. • Less 7 is acid. • greater 7 is basic (alkaline). Strong acids - role in the body ■ In strong acids all molecules dissociate. ■ HC1 is highly acidic and found only in the stomach. • H...
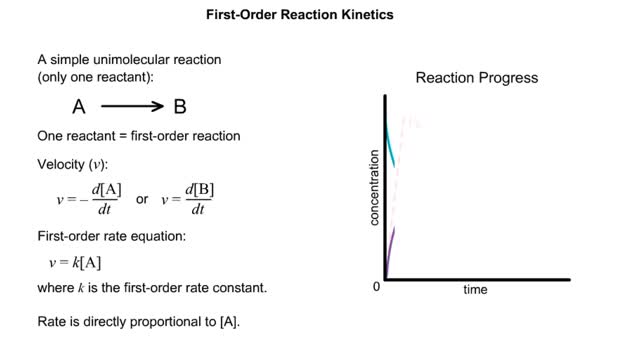
By: HWC, Views: 10236
✔ https://HomeworkClinic.com ✔ https://Videos.HomeworkClinic.com ✔ Ask questions here: https://HomeworkClinic.com/Ask Follow us: ▶ Facebook: https://www.facebook.com/HomeworkClinic ▶ Review Us: https://trustpilot.com/review/homeworkclinic.com Kinetics is the study of the rat...
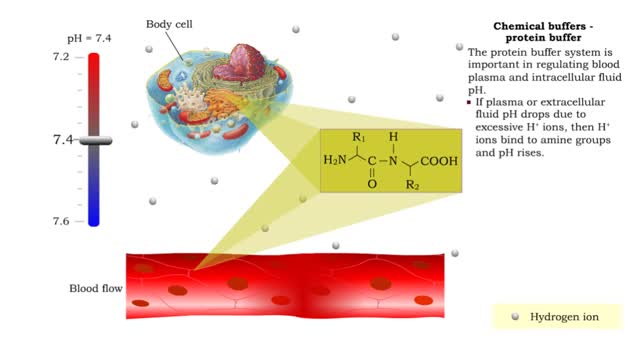
Chemical Buffers - protein buffer, phosphate buffer system and bicarbonate buffer system
By: HWC, Views: 11213
• There are a variety of chemicals in body fluids that prevent the fluids from undergoing large changes in. • These chemicals buffer or regulate fluctuations in H+ concentration. • Chemical buffers: • Bind to H+ ions when there are too many in a solution so pH remains normal. •...
Advertisement