Osmosis - water transport
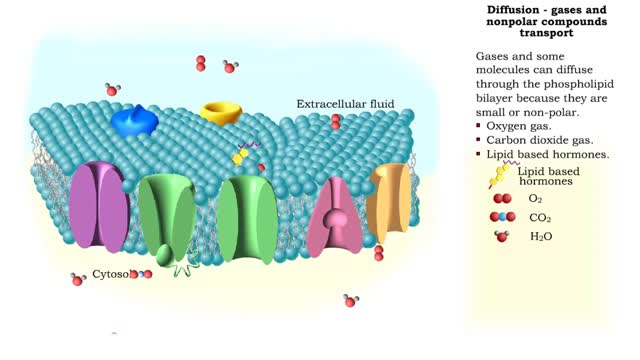
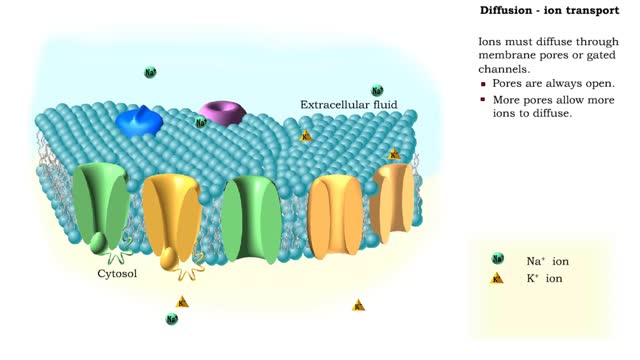
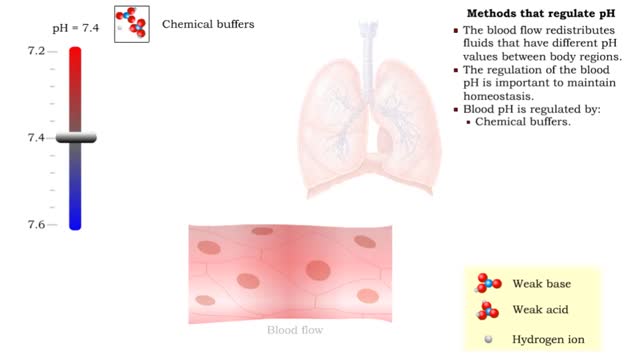
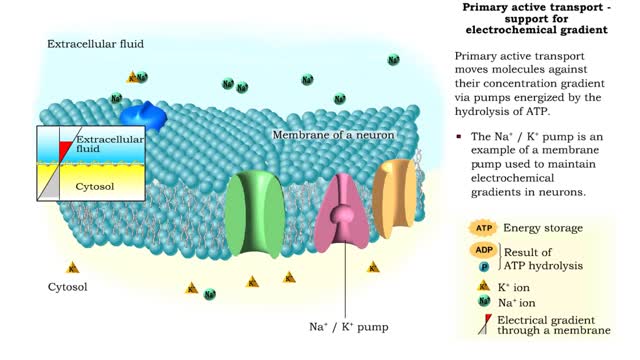
Osmosis is the flow of water down its concentration gradient, across a semi-permeable membrane. Osmosis is an example of diffusion, which is when molecules tend to distribute themselves evenly in a space. what is a semi-permeable membrane? It is a membrane or barrier that allows some molecules or substances to cross, but not others. An everyday example is the plastic wrap in your kitchen: it allows air and water vapor to travel across it, but not water or food. Depending on the direction that water flows across a plasma membrane, osmosis can cause a cell to shrink or swell. The osmotic water transport is the result of concentration differences between the two solutions separated by the membrane. Aquaporins are also known as water channels and are considered to be “the plumbing system for cells”. The aquaporins are small, very hydrophobic, intrinsic membrane proteins. Water concentration is largely determined by solute concentration. More ions results in lower water concentration in a solution. Low ions results in higher water concentration in a solution.
Add To
You must login to add videos to your playlists.
Advertisement



Comments
0 Comments total
Sign In to post comments.
No comments have been posted for this video yet.