Kinetic parameters & Kinetic experiment
By: HWC
Date Uploaded: 02/19/2020
Tags: homeworkclinic.com Homework Clinic HWC Kinetic parameters Kinetic experiment nonlinear hyperbolic fit double reciprocal plot Scatchard plot Hyperbolic graphs Kinetics rate of reaction Vmax KM
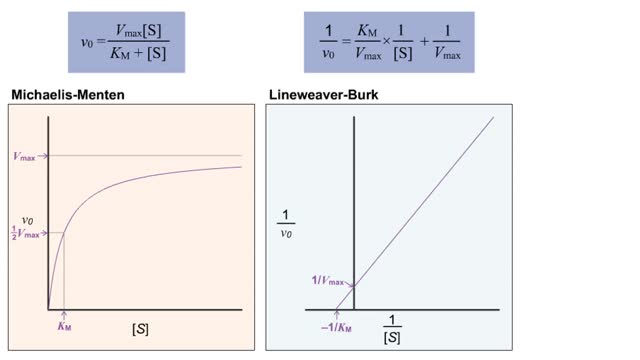
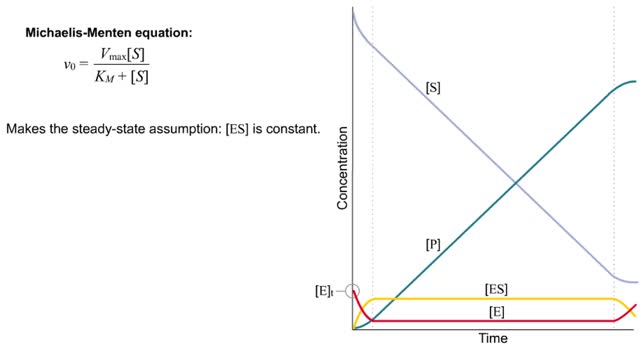
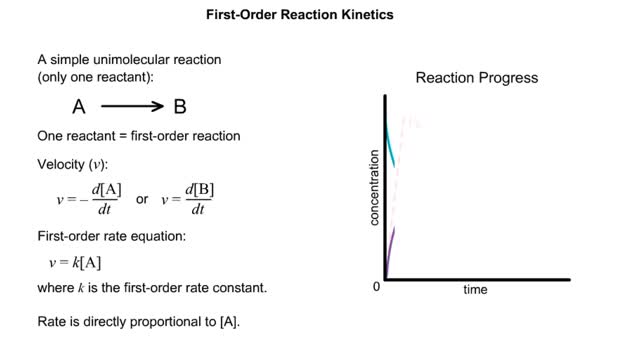
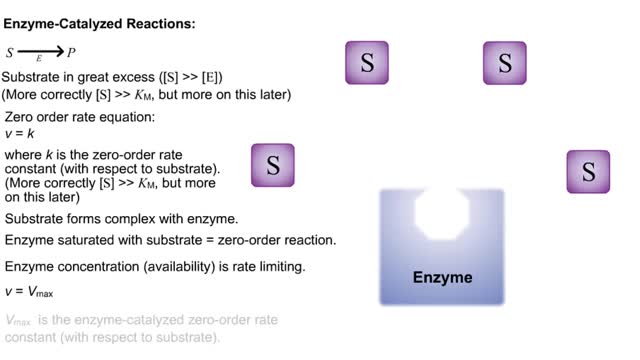
Kinetics is a measure of the speed or rate of a chemical reaction. A study of kinetics allows us to determine which variables to control (temperature, reactants, catalysts) and how to vary them in order to maximize the amount of products formed and minimize the time involved. Vmax = maximum velocity seen at enzyme-saturating [S]. KM = [S] at 1/2 Vmax. KM describes the dependence of vo on [S], and the steepness of the curve. Empirical Determination of the Relationship between [S] and vo Experimental tubes • Constant [E] in each tube. • Varying [S] added to each tube. To obtain vo, the reaction is run a short time. k-2 can be ignored. The amount of product formed over time is monitored. How can Vm and Km be determined from experimental data? From initial rate data: The most common way is to determine initial rates, v0 , from experimental values of P or S as a function of time. Hyperbolic graphs of v0 vs. [S] can be fit or transformed as we explored with the different mathematical transformations of the hyperbolic binding equation to determine Kd . These included: nonlinear hyperbolic fit double reciprocal plot Scatchard plot
Add To
You must login to add videos to your playlists.
Advertisement



Comments
0 Comments total
Sign In to post comments.
No comments have been posted for this video yet.