Chemical Equilibrium between N2O4 (colorless gas) and NO2 (brown gas)
By: HWC
Date Uploaded: 04/13/2020
Tags: homeworkclinic.com Homework Clinic HWC Chemical Equilibrium colorless gas brown gas Reversible reactions Le Chatelier’s principle Equilibrium Constant endothermic reaction exothermic reaction
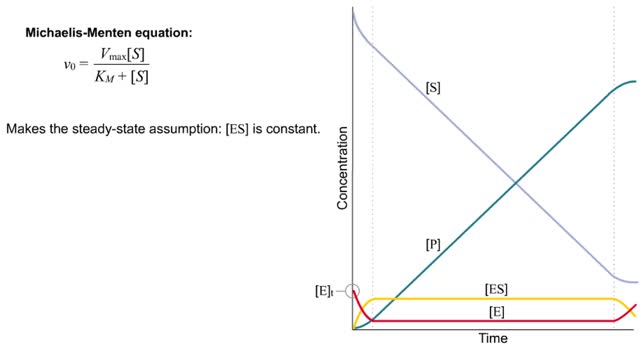
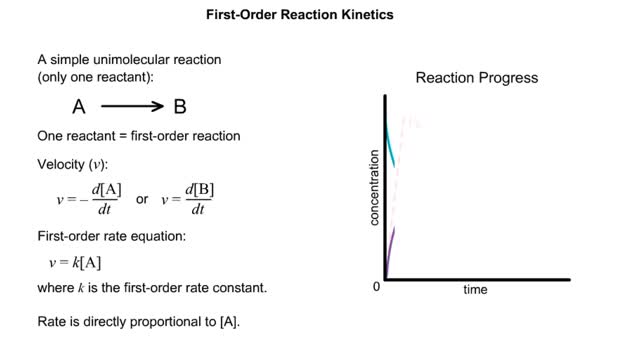
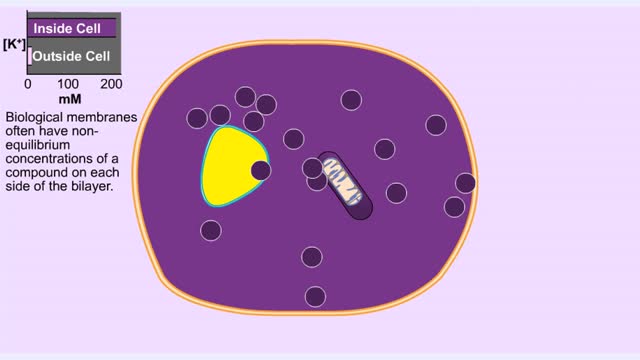
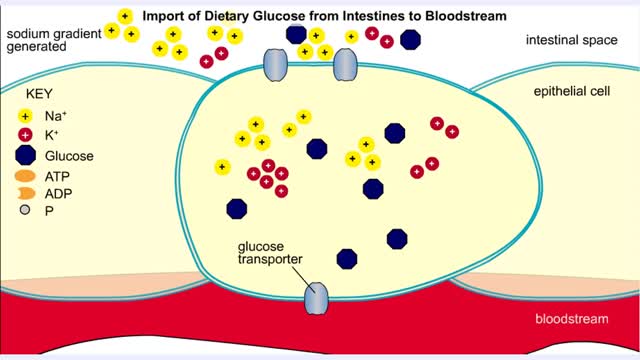
For a system at equilibrium: ◆ both forward and reverse reactions are occurring simultaneously ◆ rate of forward reaction must equal rate of reverse reaction OR Rate forward = Rate reverse ◆ concentrations of reactants and products remain constant with time the equilibrium position refers to the relative amounts of reactants and products in the system at the point of equilibrium ◆ a reaction with an equilibrium position that favors the products : [product] greater than [reactant] at equilibrium equilibrium lies to the right ◆ a reaction with an equilibrium position that favors the reactants : [reactant] greater than [product] equilibrium lies to the left
Add To
You must login to add videos to your playlists.
Advertisement



Comments
0 Comments total
Sign In to post comments.
No comments have been posted for this video yet.