Search Results
Results for: 'الروابط الأيونية'
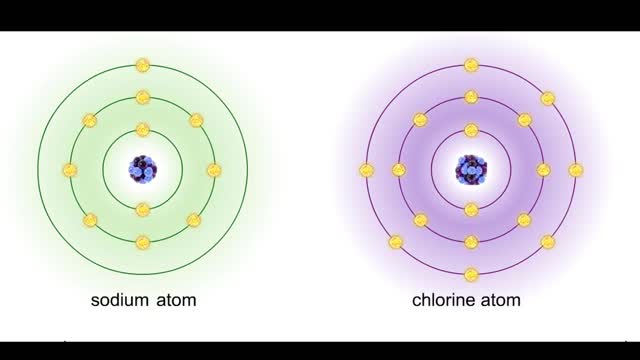
Ionic bonds - role of ions in the body
By: HWC, Views: 11625
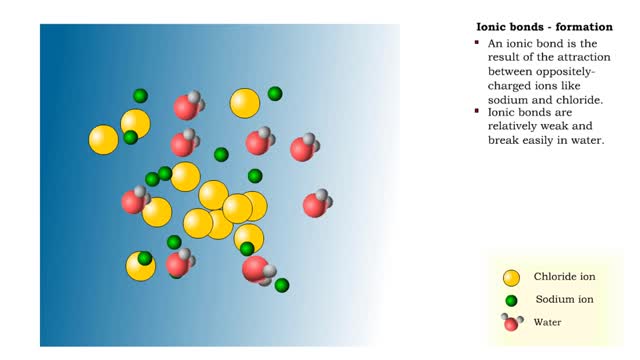
Ions • Atoms fill up the outer orbital by transferring electrons from one atom to another. • Atoms now bear a charge and are called ions. • Sodium ion, losing an electron, has a +1 charge. • Chlorine ion, gaining an electron, has a -1 charge. Formation • An ionic bond is t...
Buffers definition and the role of buffer in the body
By: HWC, Views: 11349
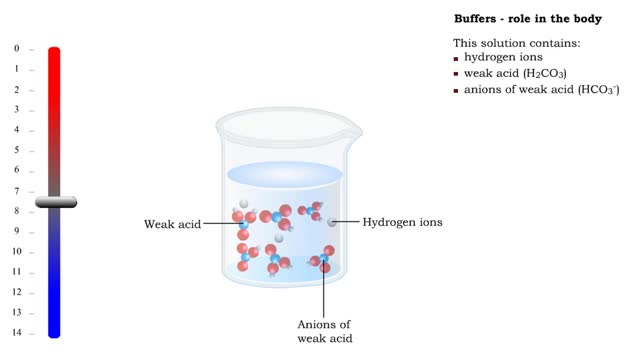
■ Too many H+ break hydrogen bonds and a protein comes apart. ■ Buffers react with excess H+ to protect proteins from breaking down. ■ Buffers consist of weak acid plus anions of that weak acid. This solution contains: • hydrogen ions • weak acid (H2CO3) • anions of we...
By: HWC, Views: 11717
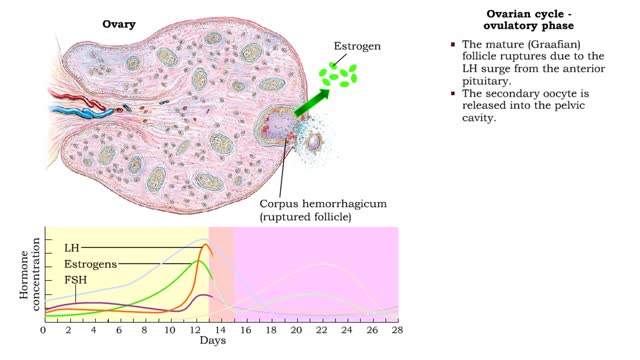
• The ovarian cycle is a monthly sequence of events, consisting of three phases: • Preovulatory • Ovulatory • Post ovulatory Preovulatory phase • prior to ovulation: Primary follicles develop into secondary follicles. • Follicular cells surrounding the primary oocyte In...
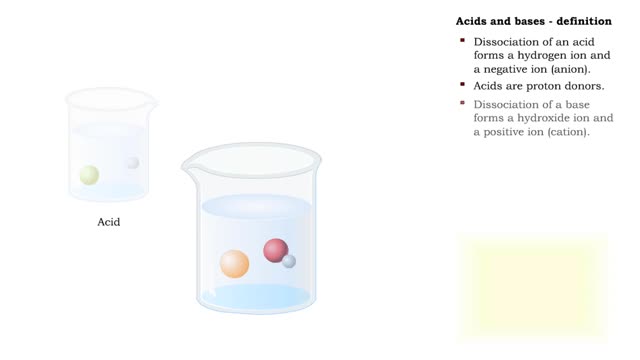
The pH scale - Strong acids and Weak acids
By: HWC, Views: 11418
The pH scale • Expresses concentration of H+. • range: 0-14. • 7 is neutral. • Less 7 is acid. • greater 7 is basic (alkaline). Strong acids - role in the body ■ In strong acids all molecules dissociate. ■ HC1 is highly acidic and found only in the stomach. • H...
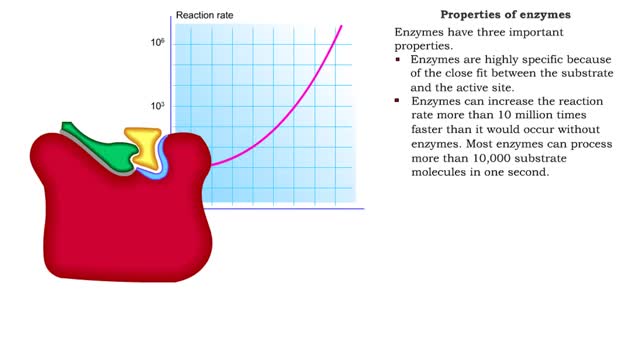
Enzyme structure - Properties of enzymes
By: HWC, Views: 11284
■ Enzymes are proteins that catalyze reactions. ■ Some enzymes have two parts: a protein or apoenzyme and a non-protein or cofactor. ■ Cofactor can be a metal ion or another organic molecule called a coenzyme. ■ Coenzymes often come from vitamins. ■ Cofactors affect the shape of...
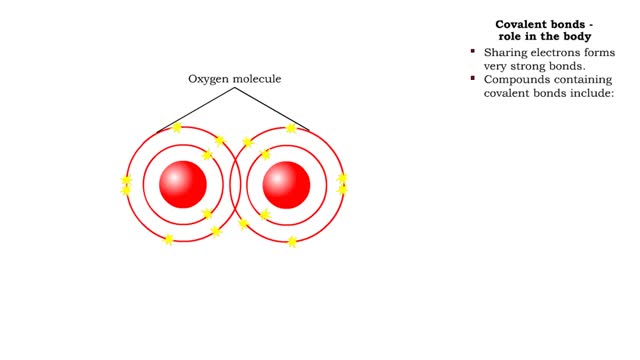
Covalent bonds - role in the body
By: HWC, Views: 11313
A covalent bond is formed when atoms share one or more pairs of electrons. This is opposed to an ionic bond, where electrons are actually transferred from one atom to another. Formation • Atoms fill up the outer orbital by sharing electrons. • Two oxygen atoms sharing electrons form on...
By: HWC, Views: 10113
The slight positive charge of a hydrogen atom in a water molecule can attract an atom with a slight negative charge, such as the nitrogen in a molecule of ammonia. This forms a hydrogen bond between the two atoms. Hydrogen bonds join the two strands of a DNA molecule. Although hydrogen bo...
By: HWC, Views: 11214
Acids and bases are found all around your house. For example, if you open your pantry or refrigerator, you might see a lot of acids. Fruit juice, soda pop, vinegar, and milk are all examples of acids. The word acid actually comes from a Latin term meaning ''sour.'' Many materials, like sugar for ...
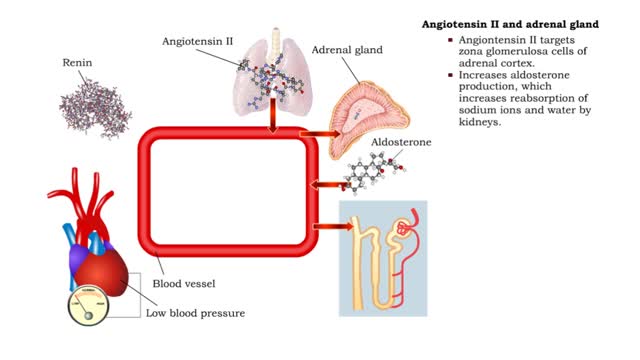
Angiotensin II - kidneys, adrenal glands and dehydration
By: HWC, Views: 11400
• Angiontensin II targets cells in the proximal convoluted tubule of the nephron. ■ The reabsorption of Na+ and Cl- ions sets up an osmotic gradient favoring the retention of water. • Decreases urine production and increases blood volume and pressure. • Angiontensin II targets zon...
Advertisement