Hydrogen bonds vs. Ionic bond
By: HWC
Date Uploaded: 02/17/2021
Tags: Hydrogen bonds vs. Ionic bond DNA molecule hydrogen bond ionic bond covalent bonds electron vacancy crystal of table salt sodium chloride روابط هيدروجين الرابطة الأيونية lien ionique liaison hydrogène enlace de hidrógeno bono iónico
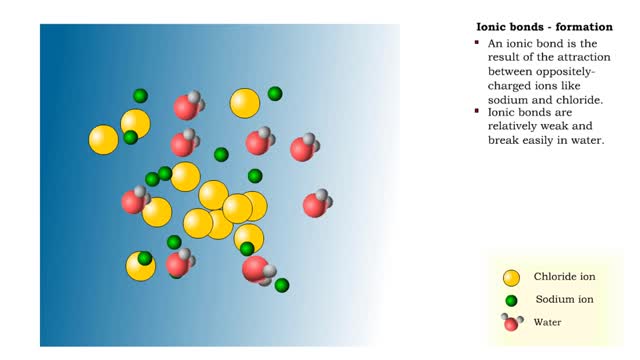
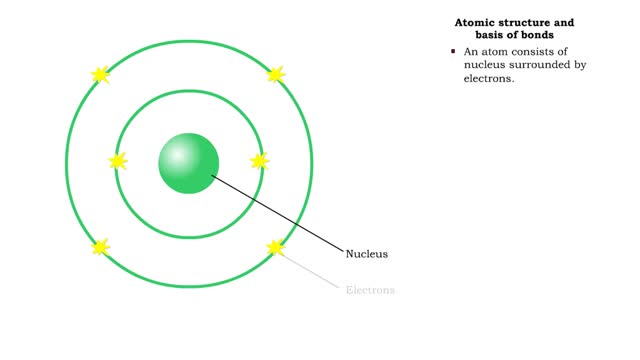
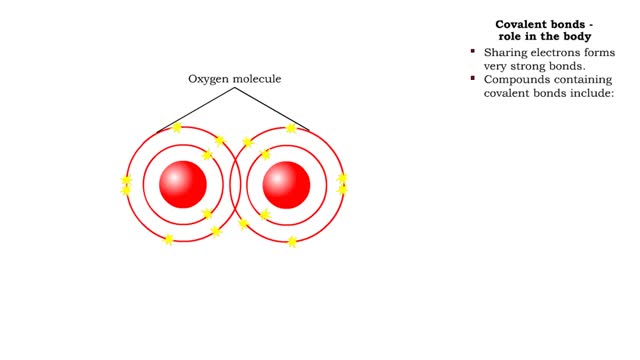
The slight positive charge of a hydrogen atom in a water molecule can attract an atom with a slight negative charge, such as the nitrogen in a molecule of ammonia. This forms a hydrogen bond between the two atoms. Hydrogen bonds join the two strands of a DNA molecule. Although hydrogen bonds are weaker than covalent bonds, extensive hydrogen bonding has a major role in the structure and behavior of DNA, water, proteins, and many other substances. Multiple hydrogen bonds can hold two large molecules together or stabilize the twists and folds of a single large protein. The lone electron in the outermost shell of a sodium atom can be pulled or knocked out. This ionizes the atoms. It is now a positively charged sodium ions. A chlorine atom has an electron vacancy in its outer shell and can acquire another electron. This ionizes the atom to form a negatively charged chloride ion. The two oppositely charged ions attract each other. An association of two ions with opposing charges is known as an ionic bond. You can observe the outcome of ionic bonding in a portion of a crystal of table salt, or sodium chloride. In such crystals, sodium ions (Nat) and chloride ions (Cl)- interact through ionic bonds.
Add To
You must login to add videos to your playlists.
Advertisement





Comments
0 Comments total
Sign In to post comments.
No comments have been posted for this video yet.