Search Results
Results for: 'Ionic bonds'
By: HWC, Views: 10113
The slight positive charge of a hydrogen atom in a water molecule can attract an atom with a slight negative charge, such as the nitrogen in a molecule of ammonia. This forms a hydrogen bond between the two atoms. Hydrogen bonds join the two strands of a DNA molecule. Although hydrogen bo...
Ionic bonds - role of ions in the body
By: HWC, Views: 11625
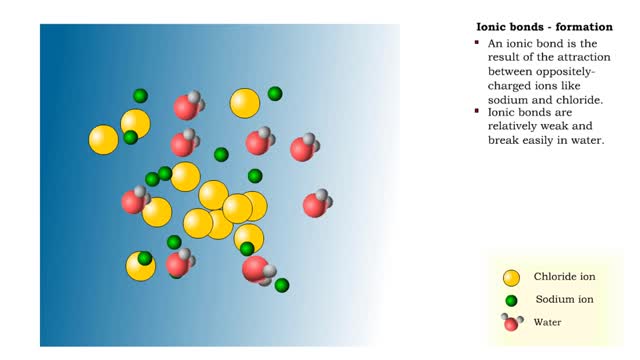
Ions • Atoms fill up the outer orbital by transferring electrons from one atom to another. • Atoms now bear a charge and are called ions. • Sodium ion, losing an electron, has a +1 charge. • Chlorine ion, gaining an electron, has a -1 charge. Formation • An ionic bond is t...
Bond types - Atomic structure and basis of bonds
By: HWC, Views: 11731
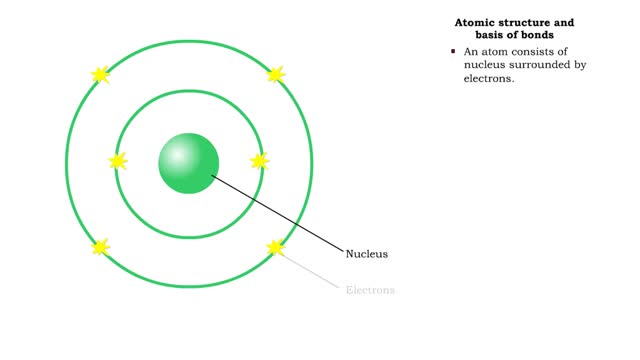
• Chemical bonds are fundamental to the structure and function of many types of molecules, such as proteins, carbohydrates, lipids, nucleic acids, gases, salts and water. ■ These molecules are composed of atoms that are held together by three different types of bonds. • The three types ...
Bond in biological molecules (Ionic, Covalent and Hydrogen bonds)/ How atoms bond?
By: HWC, Views: 8602
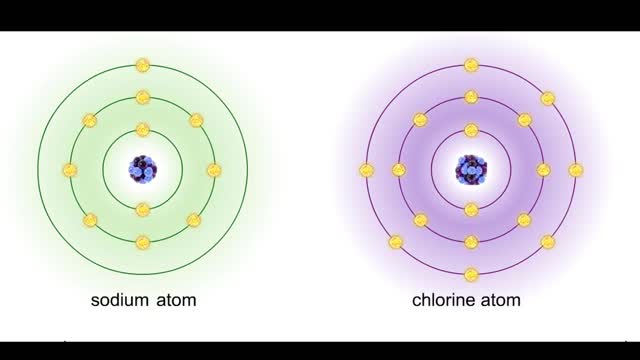
Sodium atoms and chloride atoms have unfilled orbitals in their outer shells. The lone electron in the outermost shell of a sodium atom can be pulled or knocked out. This ionizes the atom. It is now a positively charged sodium ion. A chlorine atom has an electron vacancy in its outer shell and...
Covalent bonds - role in the body
By: HWC, Views: 11312
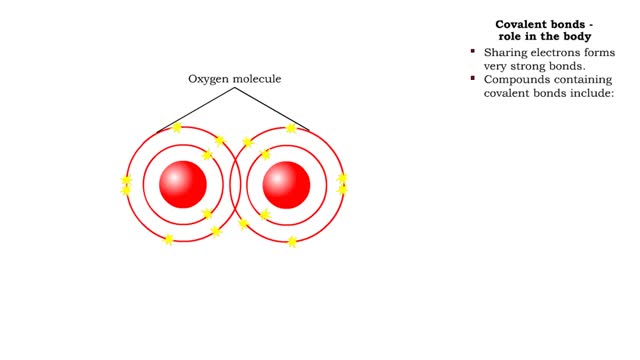
A covalent bond is formed when atoms share one or more pairs of electrons. This is opposed to an ionic bond, where electrons are actually transferred from one atom to another. Formation • Atoms fill up the outer orbital by sharing electrons. • Two oxygen atoms sharing electrons form on...
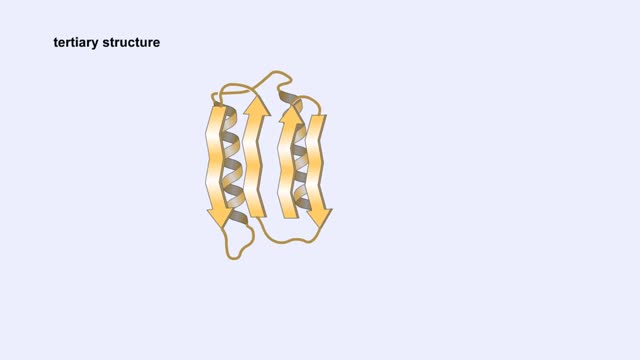
Protein Structure - Primary, Secondary, Tertiary and Quaternary
By: HWC, Views: 11187
A protein's first order structure, or primary structure, begins with the amino acid sequence of the polypeptide chain. The 20 different amino acids can be arranged in an infinite number of sequences. For example, the hormone insulin, which regulates the uptake of glucose from the blood into ce...
By: HWC, Views: 10934
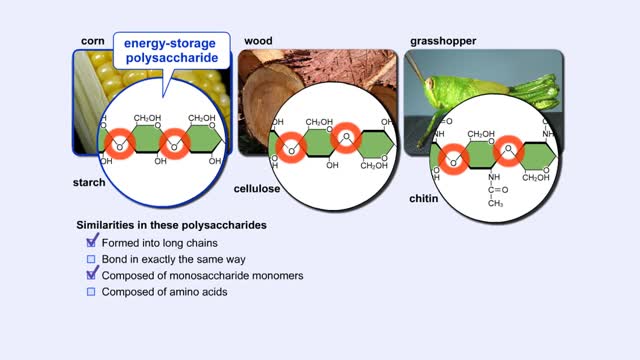
More complex sugars are called polysaccharides (from "poly" meaning "many" and "saccharum" meaning "sugar"). Many things in nature are made of polysaccharides. Here we show one of the polysaccharides in corn, another in wood, and another in the exoskeletons of insects like grasshoppers. How are a...
Hydrogen bonds - role in the body
By: HWC, Views: 11631
A hydrogen bond is the electromagnetic attraction between polar molecules in which hydrogen is bound to a larger atom, such as oxygen or nitrogen. This is not a sharing of electrons, as in a covalent bond. Instead, this is an attraction between the positive and negative poles of charged atoms. ...
By: HWC, Views: 5226
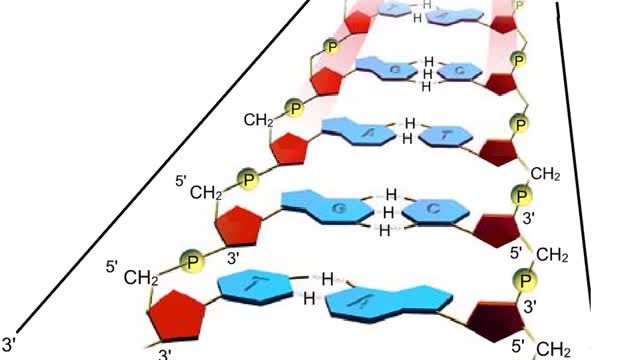
A section from a DNA double helix The backbone of each DNA strand consists of alternating deoxyribose sugars and phosphate groups. The two strands run in opposite directions. One runs from the 5' to 3' direction, the other in the 3' to 5' direction. Think of the deoxyribose units o...
Advertisement