Search Results
Results for: 'Partial negative charge'
Hydrogen bonds - role in the body
By: HWC, Views: 10813
A hydrogen bond is the electromagnetic attraction between polar molecules in which hydrogen is bound to a larger atom, such as oxygen or nitrogen. This is not a sharing of electrons, as in a covalent bond. Instead, this is an attraction between the positive and negative poles of charged atoms. ...
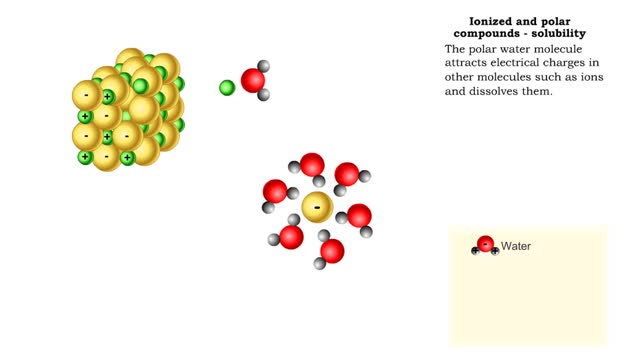
Properties of water -structure of water and polarity (Ionized and polar compounds)
By: HWC, Views: 10669
■ Water transports most of the molecules in the body. ■ The structure of a water molecule allows it to dissolve other molecules. ■ Shared electrons spend more time near the oxygen atom. ■ Oxygen end has a partial negative charge. ■ Hydrogen ends have a partial positive charge....
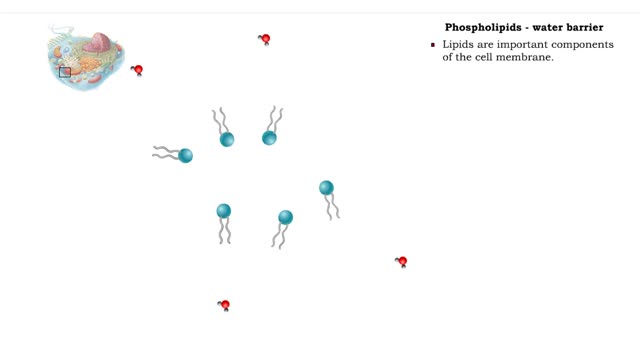
Non-polar compounds - insolubility
By: HWC, Views: 10586
• A non-polar molecule has uniform distribution of electrons. • Non-polar compounds like fatty acids in lipids have a high proportion of carbon and hydrogen. • Lipids possess no charge or partial charge. • Lipids are not attracted to water molecules. • Lipids are not soluble in...
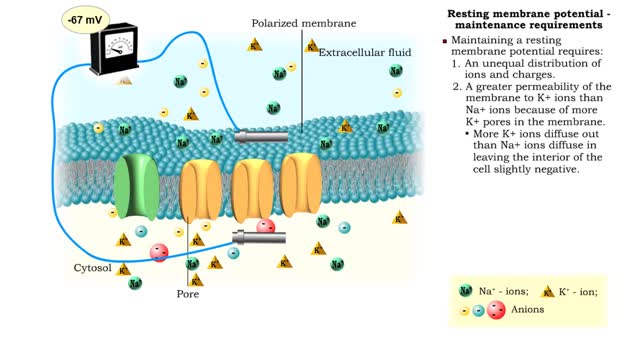
Resting membrane potential - electrical polarity and maintenance requirements
By: HWC, Views: 10199
• A resting membrane potential exists when there is a buildup of: 1. positive ions outside the membrane. 2. negative ions inside the membrane. • Membranes with opposing charges are said to be polarized. • The difference in charge applies only to the small distance across the membran...
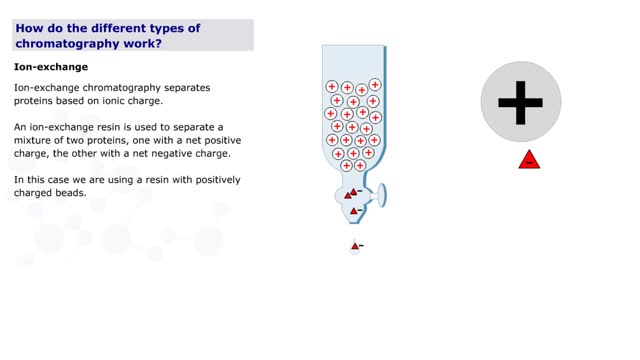
How do the different types of chromatography work? (No Audio)
By: HWC, Views: 9882
Chromatography is a term for a variety of techniques in which a mixture of dissolved components is fractionated as it moves through some type of porous matrix. A glass column is filled with beads of an inert matrix. The mixture of proteins to be purified is dissolved in a solution and passed ...
By: HWC, Views: 9990
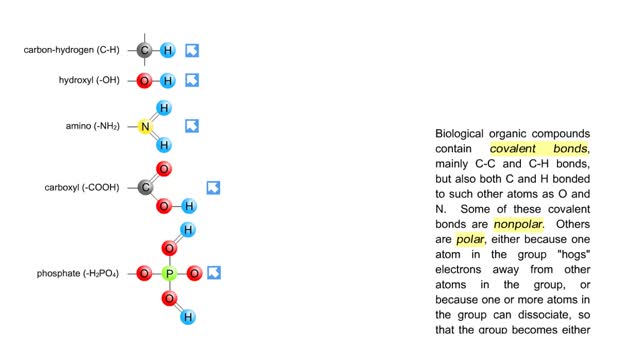
Biological organic compounds contain covalent bonds, mainly C-C and C-H bonds, but also both C and H bonded to such other atoms as O and N. Some of these covalent bonds are nonpolar. Others are polar, either because one atom in the group "hogs" electrons away from other atoms in the group, or...
Bond in biological molecules (Ionic, Covalent and Hydrogen bonds)/ How atoms bond?
By: HWC, Views: 7817
Sodium atoms and chloride atoms have unfilled orbitals in their outer shells. The lone electron in the outermost shell of a sodium atom can be pulled or knocked out. This ionizes the atom. It is now a positively charged sodium ion. A chlorine atom has an electron vacancy in its outer shell and...
Graded potentials - electrical characteristics and types
By: HWC, Views: 10737
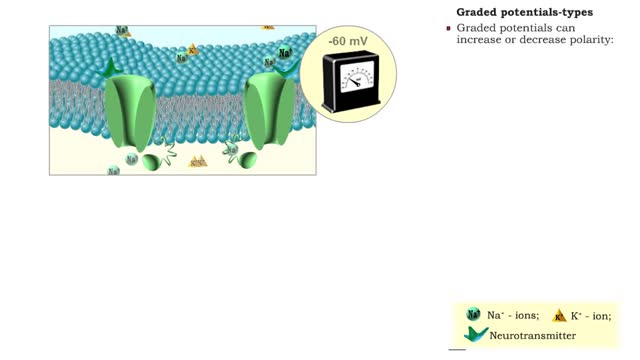
• A graded potential occurs when a gated channel is opened or closed, altering ion flow through the membrane. • Changes in ion and charge distributions cause voltage changes to the resting membrane potential. • The strength of the stimulus determines the number of gated channels affect...
Ionic bonds - role of ions in the body
By: HWC, Views: 10739
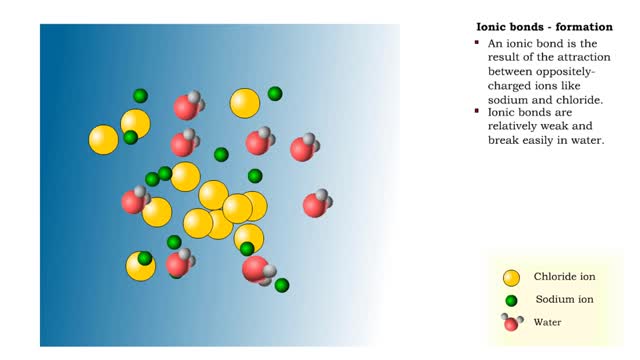
Ions • Atoms fill up the outer orbital by transferring electrons from one atom to another. • Atoms now bear a charge and are called ions. • Sodium ion, losing an electron, has a +1 charge. • Chlorine ion, gaining an electron, has a -1 charge. Formation • An ionic bond is t...
Advertisement