Search Results
Results for: 'acidic ketone'
Lipid catabolism ( ketogenesis and oxidation of glycerol) and Lipid anabolism (lipogenesis)
By: HWC, Views: 10810
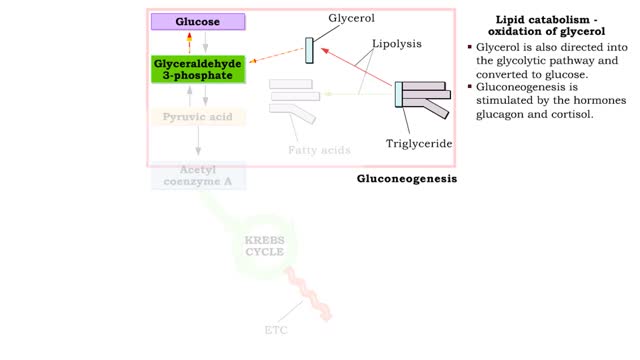
• During excessive beta oxidation, the two-carbon fatty acid fragments are converted into acidic ketone bodies. • Ketosis, the overproduction of ketone bodies, can lead to acidosis (ketoacidosis) of the blood. • After lipolysis, glycerol is converted to pyruvic acid. • Pyruvic aci...
Protein catabolism (Krebs cycle) and Protein anabolism (protein synthesis)
By: HWC, Views: 10977
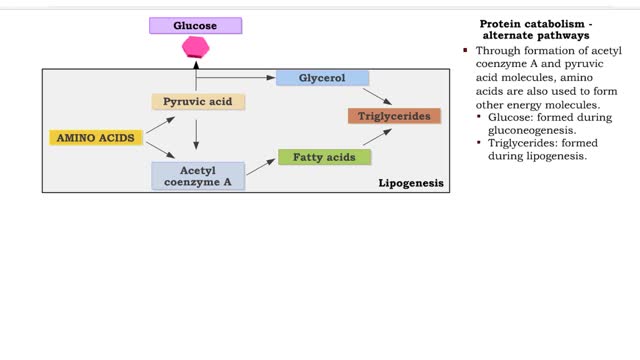
• Deaminated acids are brought into the Krebs cycle to be oxidized to CO2 and H2O. • Before entering the Krebs cycle, the deaminated acids are converted into intermediate products (pyruvic acid, acetyl coenzyme A, carbonic acids). • In the Krebs cycle, amino acids are oxidized to form r...
By: HWC, Views: 9986
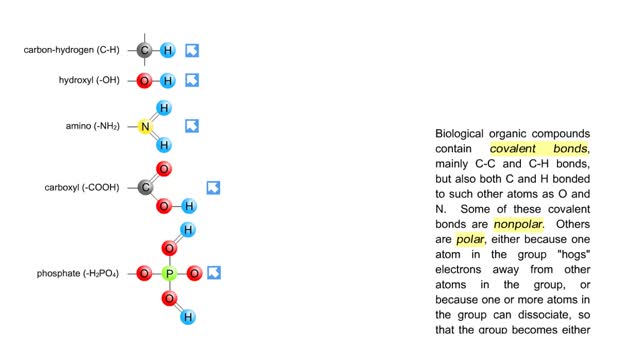
Biological organic compounds contain covalent bonds, mainly C-C and C-H bonds, but also both C and H bonded to such other atoms as O and N. Some of these covalent bonds are nonpolar. Others are polar, either because one atom in the group "hogs" electrons away from other atoms in the group, or...
The pH scale - Strong acids and Weak acids
By: HWC, Views: 10575
The pH scale • Expresses concentration of H+. • range: 0-14. • 7 is neutral. • Less 7 is acid. • greater 7 is basic (alkaline). Strong acids - role in the body ■In strong acids all molecules dissociate. ■HC1 is highly acidic and found only in the stomach. • H...
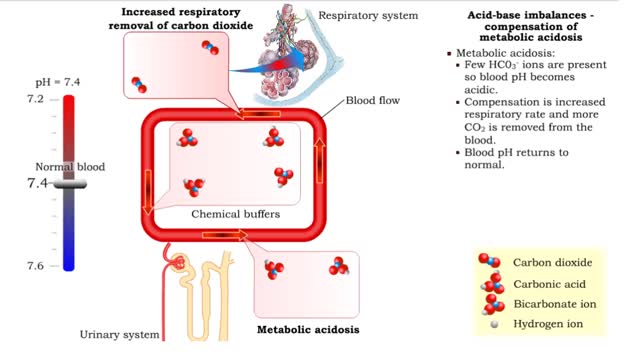
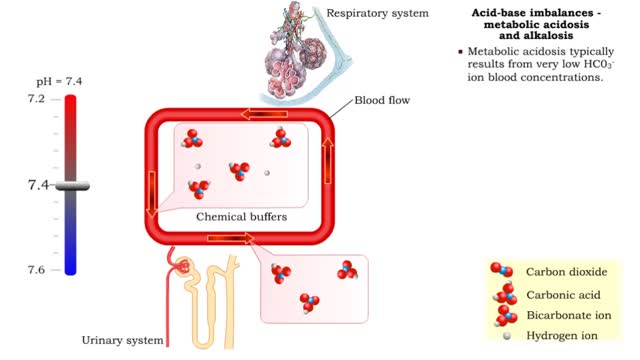
Acid-base imbalances - compensation of metabolic acidosis and alkalosis
By: HWC, Views: 10705
1. Metabolic acidosis: • Few HC03- ions are present so blood pH becomes acidic. • Compensation is increased respiratory rate and more CO2 is removed from the blood. • Blood pH returns to normal. 2. Metabolic alkalosis: • Many HC03- ions are present so blood pH becomes alkaline...
Acid-base imbalances - compensation of respiratory acidosis and alkalosis
By: HWC, Views: 10675
• When one pH balancing system is affected then the other balancing system attempts to correct, or compensate for, the pH imbalance. - Respiratory acidosis: • Excessive CO2 is present so blood pH becomes acidic. • Compensation is increased secretion of H+ into urine and reabsorption ...
By: Administrator, Views: 15078
Hyperglycemia means high (hyper) glucose (gly) in the blood (emia). Your body needs glucose to properly function. Your cells rely on glucose for energy. Hyperglycemia is a defining characteristic of diabetes—when the blood glucose level is too high because the body isn't properly using or doesn...
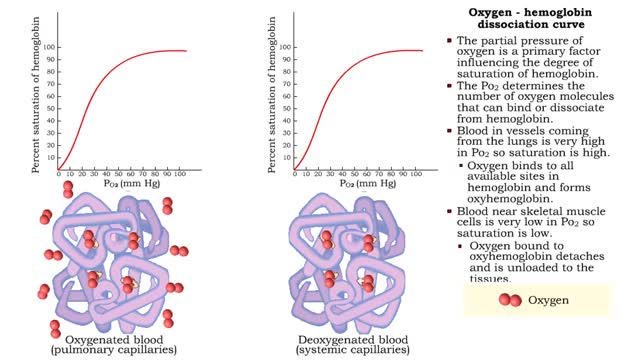
Oxygen - hemoglobin dissociation curve & Hemoglobin's affinity with oxygen - acidity
By: HWC, Views: 11102
• The partial pressure of oxygen is a primary factor influencing the degree of saturation of hemoglobin. • The Po2 determines the number of oxygen molecules that can bind or dissociate from hemoglobin. • Blood in vessels coming from the lungs is very high in Po2 so saturation is high. ...
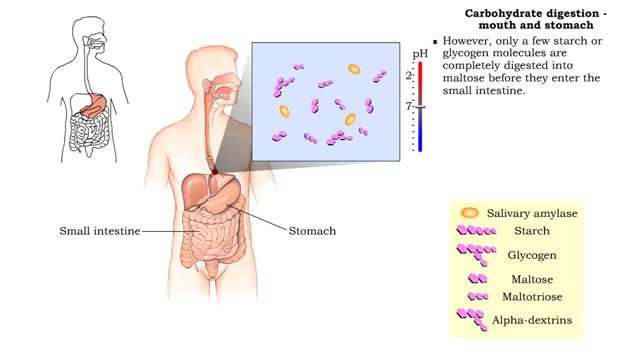
Carbohydrate digestion - mouth and stomach & pancreas and small intestine
By: HWC, Views: 10311
• Digestion of complex carbohydrates (starches and glycogen) involves: • Amylases produced by the salivary glands and pancreas. • Brush-border enzymes in small intestine. • In the mouth, amylase from the parotid and submandibular salivary glands begins carbohydrate digestion. â€...
Advertisement