Search Results
Results for: 'toxic ammonium ions'
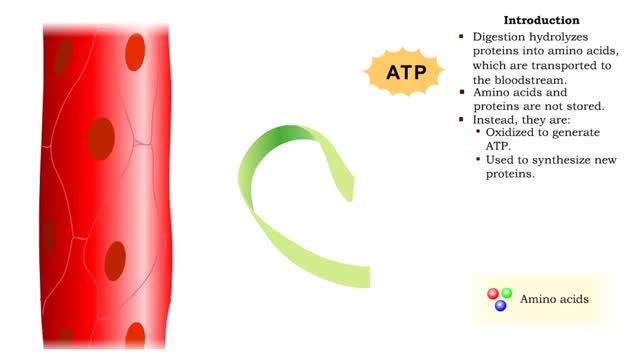
Protein catabolism - deamination
By: HWC, Views: 11365
• Digestion hydrolyzes proteins into amino acids, which are transported to the bloodstream. • Amino acids and proteins are not stored. • Instead, they are: • Oxidized to generate ATP. • Used to synthesize new proteins. • Converted to carbohydrates or lipids for storage (if e...
By: Administrator, Views: 14581
How smoking triggers asthma attacks and respiratory issues. Secondhand smoke is smoke from burning tobacco products, such as cigarettes, cigars, or pipes. Secondhand smoke also is smoke that has been exhaled, or breathed out, by the person smoking. Tobacco smoke contains more than 7,000 ...
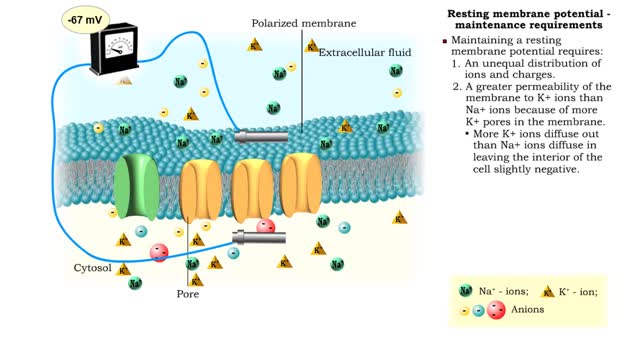
Resting membrane potential - electrical polarity and maintenance requirements
By: HWC, Views: 10815
• A resting membrane potential exists when there is a buildup of: 1. positive ions outside the membrane. 2. negative ions inside the membrane. • Membranes with opposing charges are said to be polarized. • The difference in charge applies only to the small distance across the membran...
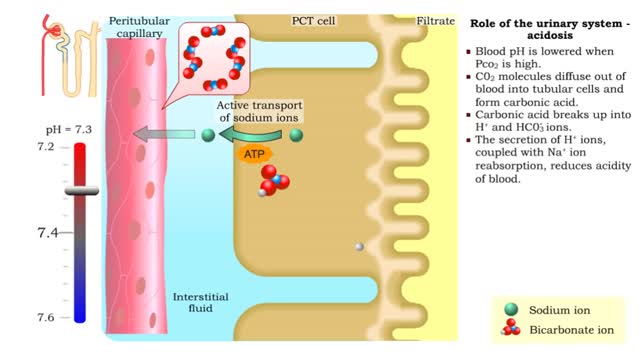
Role of the urinary system - acidosis and alkalosis
By: HWC, Views: 11309
• Tubular cells of the proximal convoluted tubule and collecting tubules can alter filtrate pH and therefore blood pH. • These cells can affect blood pH with two coupled mechanisms: • Reabsorption of bicarbonate ions. • Secretion of hydrogen ions. • The reabsorption of bicarbonate...
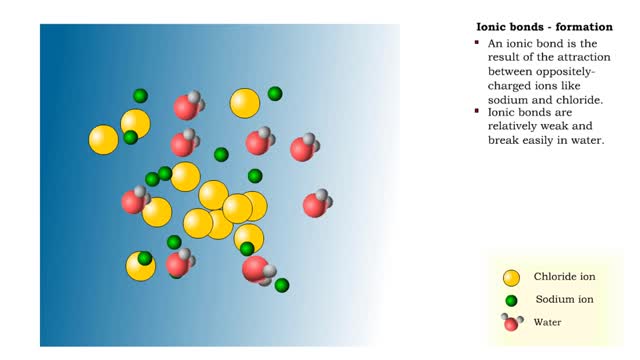
Ionic bonds - role of ions in the body
By: HWC, Views: 11443
Ions • Atoms fill up the outer orbital by transferring electrons from one atom to another. • Atoms now bear a charge and are called ions. • Sodium ion, losing an electron, has a +1 charge. • Chlorine ion, gaining an electron, has a -1 charge. Formation • An ionic bond is t...
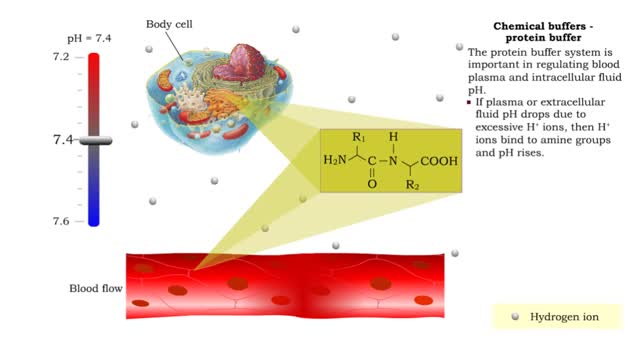
Chemical Buffers - protein buffer, phosphate buffer system and bicarbonate buffer system
By: HWC, Views: 11350
• There are a variety of chemicals in body fluids that prevent the fluids from undergoing large changes in. • These chemicals buffer or regulate fluctuations in H+ concentration. • Chemical buffers: • Bind to H+ ions when there are too many in a solution so pH remains normal. •...
What are Strong & Weak Acids and How they're different?
By: HWC, Views: 9943
Let's consider the changes that take place when hydrogen chloride, HCI, is added to water. You will need to recognize space-filling models of HCI molecules, hydronium ions (H30+), chloride ions (C11, and water molecules (H20). They are shown at the right. When HC1 molecules dissolve in water, ...
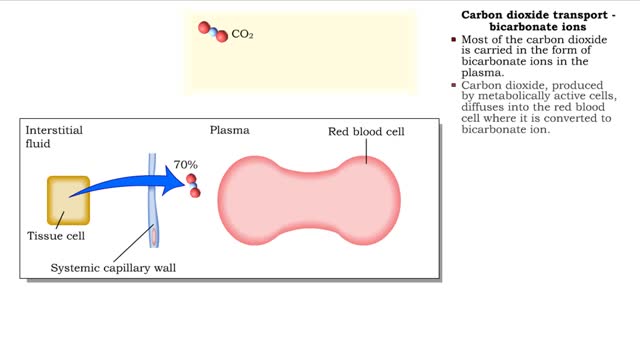
Methods of carbon dioxide transport - carbaminohemoglobin and bicarbonate ions
By: HWC, Views: 11209
• Carbon dioxide is transported three ways: • As bicarbonate ions in the plasma. • Bound to hemoglobin. • As a dissolved gas in the plasma. • A small percent of carbon dioxide is transported as a dissolved gas. • Some of the carbon dioxide is bound to hemoglobin, in the fo...
Advertisement