Search Results
Results for: 'MS'
By: HWC, Views: 11543
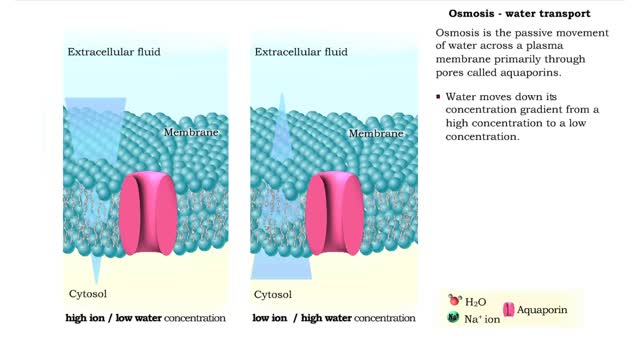
Osmosis is the flow of water down its concentration gradient, across a semi-permeable membrane. Osmosis is an example of diffusion, which is when molecules tend to distribute themselves evenly in a space. what is a semi-permeable membrane? It is a membrane or barrier that allows some molec...
Primary Active Transport - electrochemical gradient and ion transport / water movement
By: HWC, Views: 11577
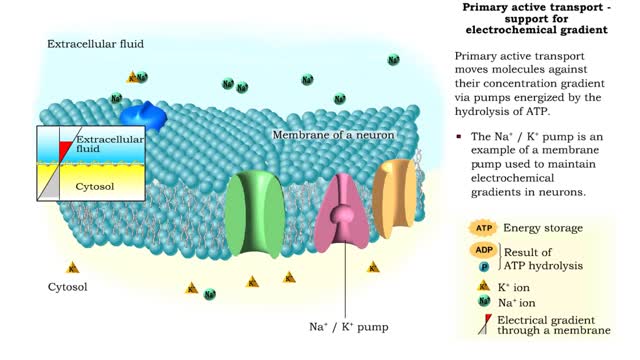
Energy derived from ATP changes the shape of a transporter protein which pumps a substance across a plasma membrane against its concentration gradient An electrochemical gradient is a gradient of electrochemical potential, usually for an ion that can move across a membrane. The gradient consis...
By: HWC, Views: 11997
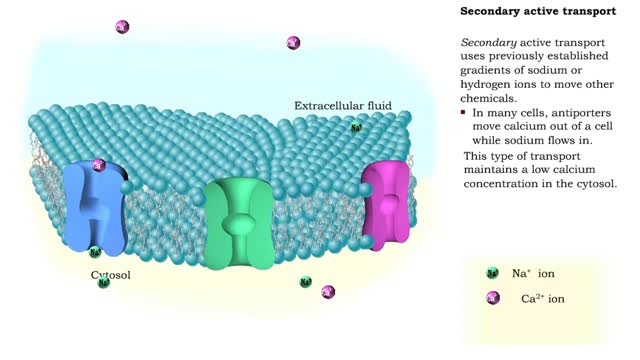
Energy stored (in a hydrogen or sodium concentration gradient) is used to drive other substances against their own concentration gradients Secondary active transport, is transport of molecules across the cell membrane utilizing energy in other forms than ATP. In many cells, antiporters mov...
Bone tissue types - compact and spongy
By: HWC, Views: 11394
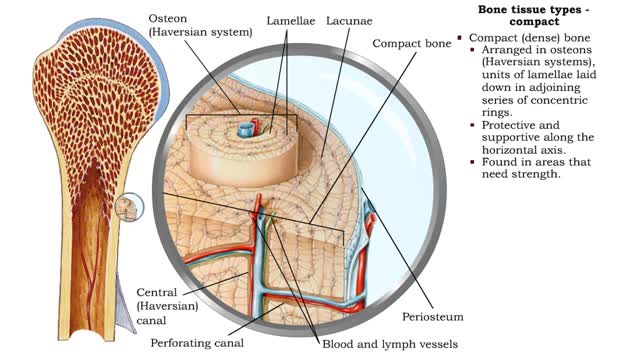
Bone tissue types • There are two types of bone tissue: compact and spongy. • All the bones of the skeleton have both kinds of bone tissue. • Compact (dense) bone • Arranged in osteons (Haversian systems), units of lamellae laid down in adjoining series of concentric rings. • P...
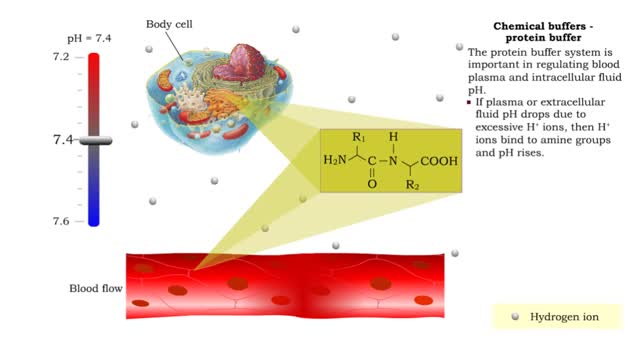
Chemical Buffers - protein buffer, phosphate buffer system and bicarbonate buffer system
By: HWC, Views: 11587
• There are a variety of chemicals in body fluids that prevent the fluids from undergoing large changes in. • These chemicals buffer or regulate fluctuations in H+ concentration. • Chemical buffers: • Bind to H+ ions when there are too many in a solution so pH remains normal. •...
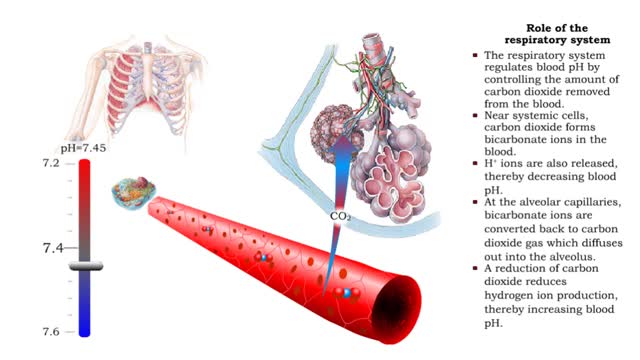
Role of the respiratory system - effect of altered ventilation rates
By: HWC, Views: 11985
• The respiratory system regulates blood pH by controlling the amount of carbon dioxide removed from the blood. • Near systemic cells, carbon dioxide forms bicarbonate ions in the blood. H+ ions are also released, thereby decreasing blood pH. • At the alveolar capillaries, bicarbonate io...
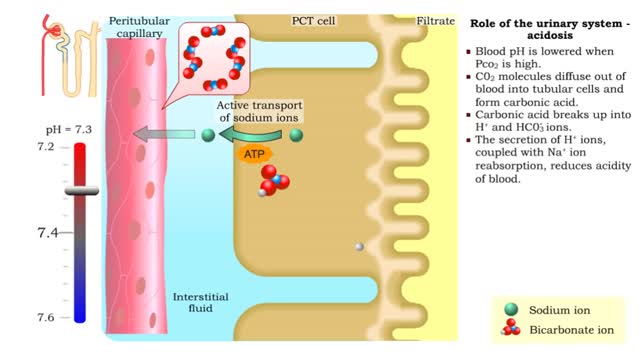
Role of the urinary system - acidosis and alkalosis
By: HWC, Views: 11546
• Tubular cells of the proximal convoluted tubule and collecting tubules can alter filtrate pH and therefore blood pH. • These cells can affect blood pH with two coupled mechanisms: • Reabsorption of bicarbonate ions. • Secretion of hydrogen ions. • The reabsorption of bicarbonate...
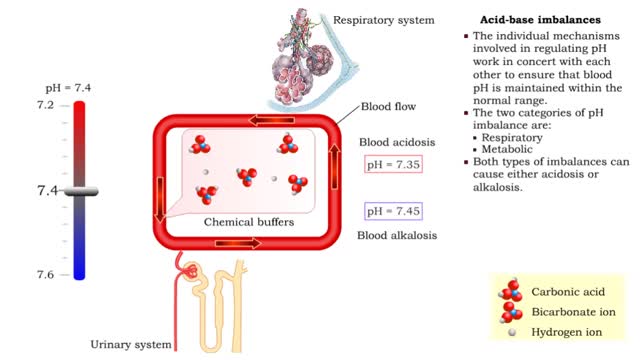
Acid-base imbalances - respiratory acidosis and alkalosis
By: HWC, Views: 11729
• The individual mechanisms involved in regulating pH work in concert with each other to ensure that blood pH is maintained within the normal range. • The two categories of pH imbalance are: • Respiratory • Metabolic • Both types of imbalances can cause either acidosis or alka...
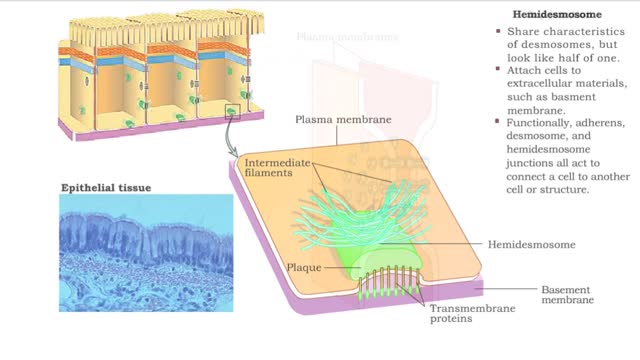
Type of Cell Junctions - Desmosome, Hemidesmosomes and Gap Junctions
By: HWC, Views: 11752
Cell Junctions: Cell junctions are found in some multi-cellular organisms. They exist of complexes and are found between cells and between cells and other structures. The junctions provide a way for cells to connect and exchange signals. What are tight junctions, desmosomes, and gap junctions...
Advertisement