Search Results
Results for: 'covalent bonds'
Peptide Bond Formation Animation
By: HWC, Views: 5550
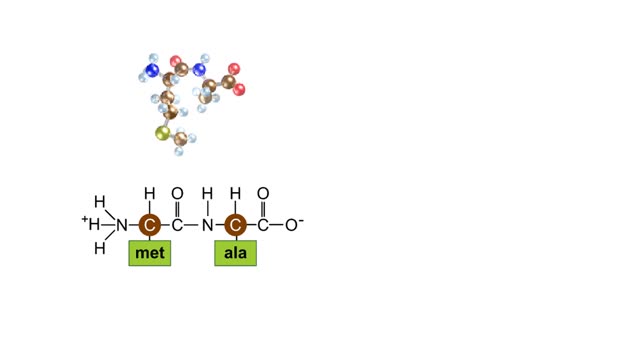
During protein synthesis, peptide bonds link amino acids together in the order specified by DNA instructions. In this case, the first two amino acids in the protein are methionine and alanine. Here are ball-and-stick models of these amino acids. Peptide bond formation is a type of condensatio...
By: HWC, Views: 11327
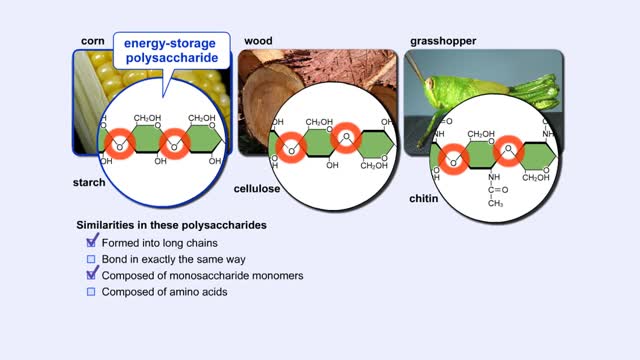
More complex sugars are called polysaccharides (from "poly" meaning "many" and "saccharum" meaning "sugar"). Many things in nature are made of polysaccharides. Here we show one of the polysaccharides in corn, another in wood, and another in the exoskeletons of insects like grasshoppers. How are a...
By: HWC, Views: 5638
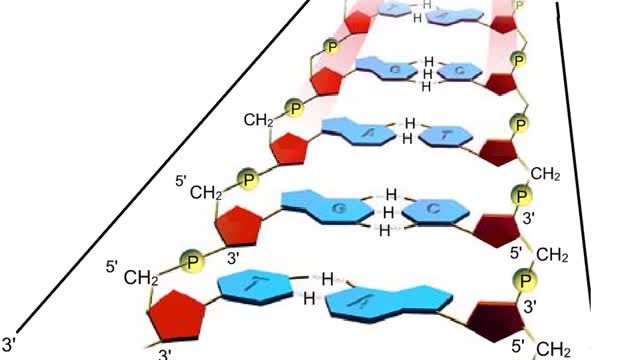
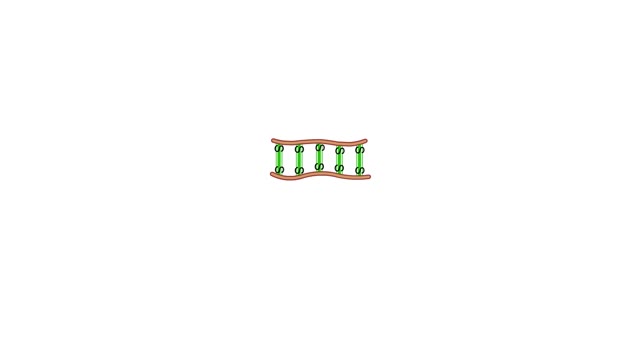
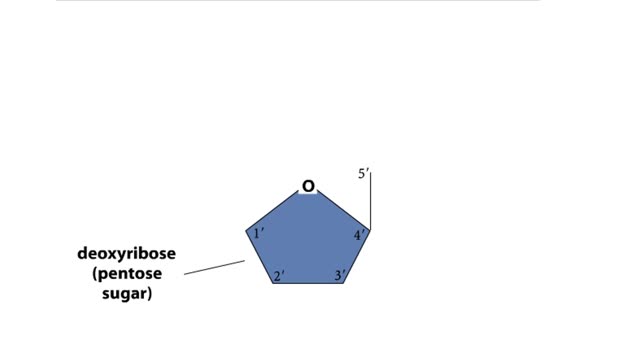
A section from a DNA double helix The backbone of each DNA strand consists of alternating deoxyribose sugars and phosphate groups. The two strands run in opposite directions. One runs from the 5' to 3' direction, the other in the 3' to 5' direction. Think of the deoxyribose units o...
Protein catabolism (Krebs cycle) and Protein anabolism (protein synthesis)
By: HWC, Views: 12321
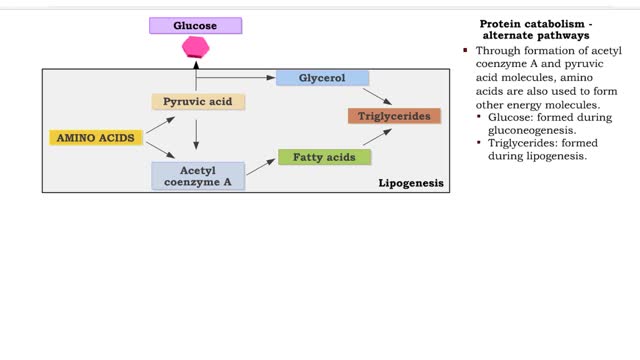
• Deaminated acids are brought into the Krebs cycle to be oxidized to CO2 and H2O. • Before entering the Krebs cycle, the deaminated acids are converted into intermediate products (pyruvic acid, acetyl coenzyme A, carbonic acids). • In the Krebs cycle, amino acids are oxidized to form r...
Anatomy and Chemical Makeup of a Single Hair (Animation)
By: HWC, Views: 10103
The hair's outer cuticle surrounds hair cells filled with tough keratin macrofibrils. Each macrofibril consists of smaller microfibrils. A microfibril is made up of three keratin polypeptide chains. The chains are linked together by disulfide bonds. A hair consists of keratin chains held...
Activation Energy - Valence Electrons
By: HWC, Views: 11187
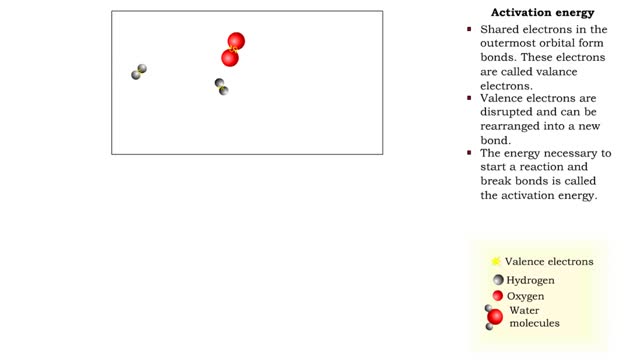
■ Shared electrons in the outermost orbital form bonds. These electrons are called valence electrons. ■ Valence electrons are disrupted and can be rearranged into a new bond. ■ The energy necessary to start a reaction and break bonds is called the activation energy. ■ Reactants have...
Ionic bonds - role of ions in the body
By: HWC, Views: 11985
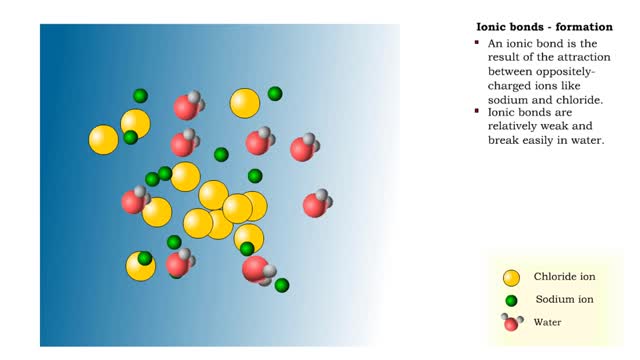
Ions • Atoms fill up the outer orbital by transferring electrons from one atom to another. • Atoms now bear a charge and are called ions. • Sodium ion, losing an electron, has a +1 charge. • Chlorine ion, gaining an electron, has a -1 charge. Formation • An ionic bond is t...
Structure of Amino Acid, Peptide Bonds & Polypeptides
By: HWC, Views: 11299
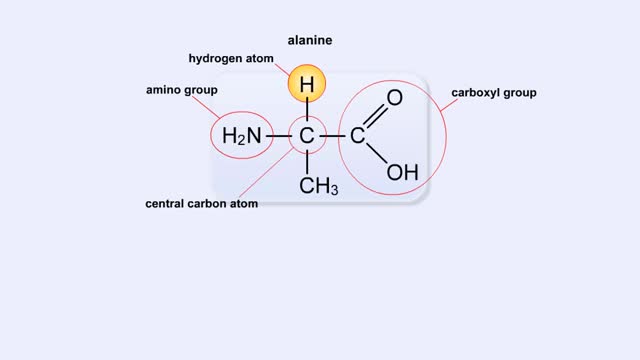
Here are the molecular formulas of three different amino acids. All amino acids share this backbone. The main difference between every amino acid is the side groups seen here, and these side groups give each of the amino acids their different characteristics. But before we get into that, let's ...
Major Elements in Biological Molecules: Nucleic acids
By: HWC, Views: 11612
DNA and RNA are nucleic acids (polymers of nucleotides). Two polymers with complementary nucleotide sequences can pair with each other. This pairing endows nucleic acids with the ability to store, transmit, and retrieve genetic information. Two strands of DNA pair by hydrogen bonding. A compon...
Advertisement