Search Results
Results for: 'organic molecule'
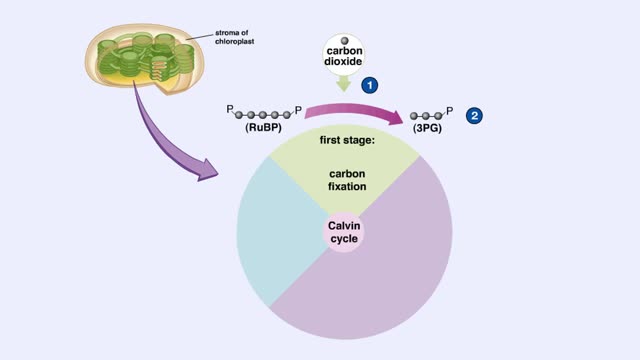
Calvin cycle (The light-independent reactions )
By: HWC, Views: 10527
The light-independent reactions of photosynthesis occur in the stroma of the chloroplast. Carbon dioxide enters the leaf through tiny pores or stomata and diffuses into the chloroplast. The first stage of the Calvin cycle is the attachment of a carbon dioxide molecule to a 5-carbon ribulose bi...
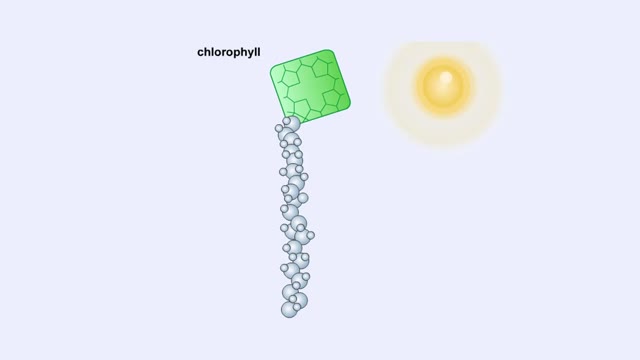
Electromagnetic Spectrum, Chlorophyll and Pigment & Light
By: HWC, Views: 10482
The sun gives off radiation that is called the electromagnetic spectrum. This is energy that travels as wavelengths and includes radio waves, X-rays and ultraviolet light. A portion of this radiation is known as visible light, and is the type of radiation that plants use to manufacture sugars. ...
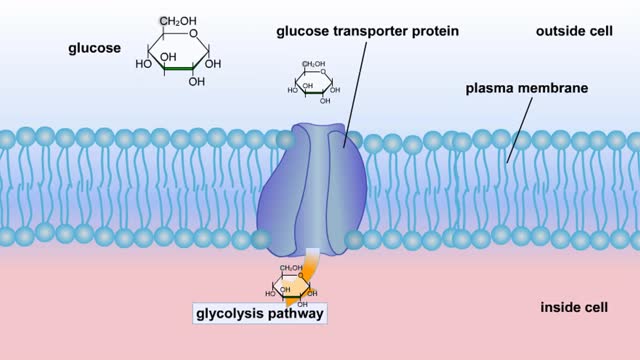
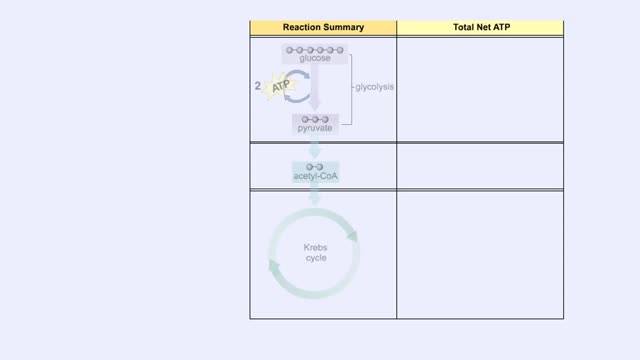
Cellular Respiration & Glucose Mobilization (Glucose transport & Phosphorylation of Glucose)
By: HWC, Views: 10477
Glucose is completely broken down into CO2 and H2O during the process of cellular respiration, which includes 3 stages: 1) glycolysis; 2) the Krebs Cycle; and 3) the electron transport chain. Glucose enters this energy yielding pathway of cellular respiration in the first stage known as...
ETC Protein Complexes & Chemiosmosis (Total ATP Production and ATP Synthase)
By: HWC, Views: 10417
You will notice that FADH2 donates two electrons further downstream than NADH. This results in only two protons being pumped across the inner membrane. The final electron acceptor for these transported electrons is oxygen. Oxygen receives these electrons, plus protons from the aqueous matrix. ...
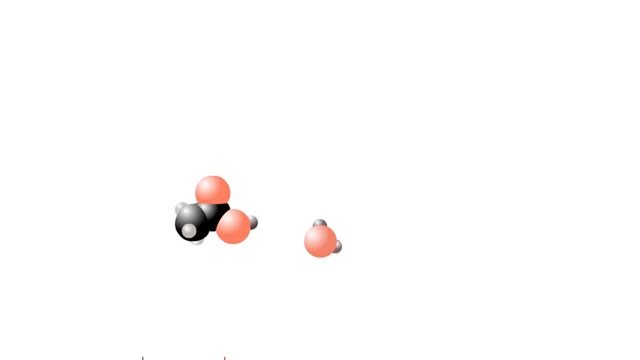
What are Strong & Weak Acids and How they're different?
By: HWC, Views: 9498
Let's consider the changes that take place when hydrogen chloride, HCI, is added to water. You will need to recognize space-filling models of HCI molecules, hydronium ions (H30+), chloride ions (C11, and water molecules (H20). They are shown at the right. When HC1 molecules dissolve in water, ...
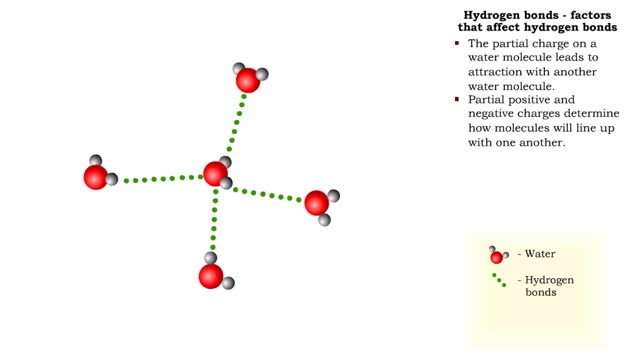
By: HWC, Views: 9492
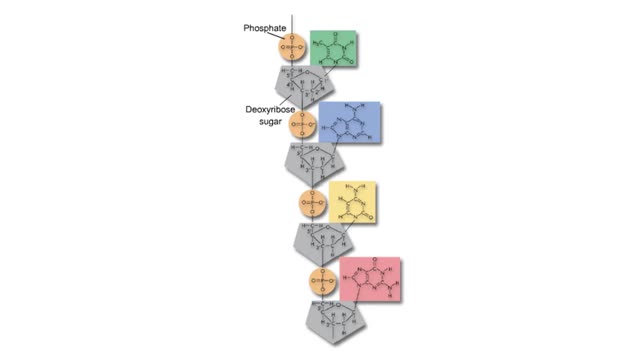
The slight positive charge of a hydrogen atom in a water molecule can attract an atom with a slight negative charge, such as the nitrogen in a molecule of ammonia. This forms a hydrogen bond between the two atoms. Hydrogen bonds join the two strands of a DNA molecule. Although hydrogen bo...
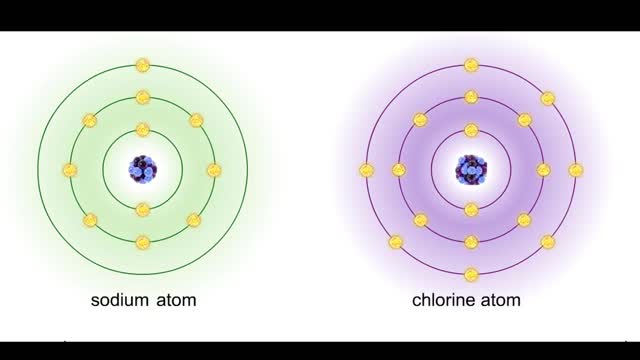
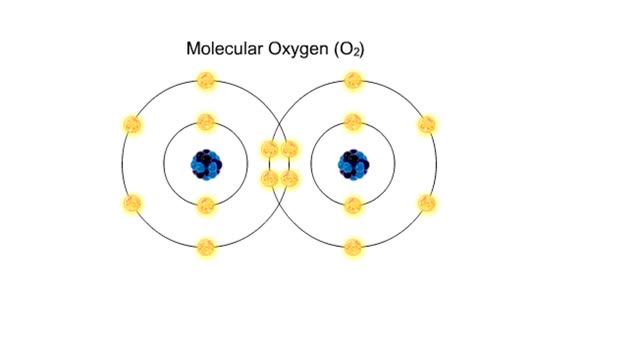
Bond in biological molecules (Ionic, Covalent and Hydrogen bonds)/ How atoms bond?
By: HWC, Views: 8025
Sodium atoms and chloride atoms have unfilled orbitals in their outer shells. The lone electron in the outermost shell of a sodium atom can be pulled or knocked out. This ionizes the atom. It is now a positively charged sodium ion. A chlorine atom has an electron vacancy in its outer shell and...
Hydrogen bonds - role in the body
By: HWC, Views: 11014
A hydrogen bond is the electromagnetic attraction between polar molecules in which hydrogen is bound to a larger atom, such as oxygen or nitrogen. This is not a sharing of electrons, as in a covalent bond. Instead, this is an attraction between the positive and negative poles of charged atoms. ...
Advertisement