Search Results
Results for: 'partial charge'
Ionic bonds - role of ions in the body
By: HWC, Views: 11965
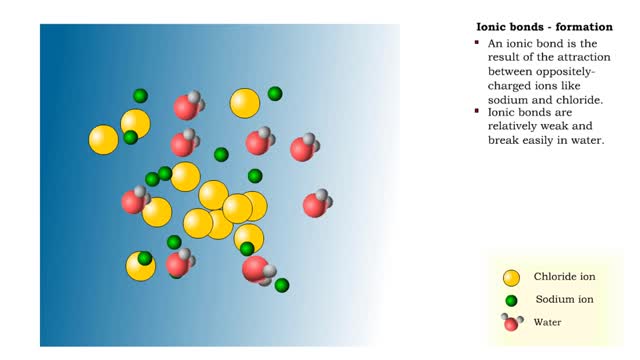
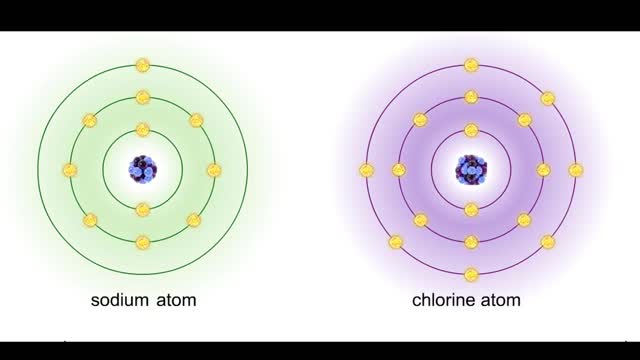
Ions • Atoms fill up the outer orbital by transferring electrons from one atom to another. • Atoms now bear a charge and are called ions. • Sodium ion, losing an electron, has a +1 charge. • Chlorine ion, gaining an electron, has a -1 charge. Formation • An ionic bond is t...
Bond in biological molecules (Ionic, Covalent and Hydrogen bonds)/ How atoms bond?
By: HWC, Views: 8971
Sodium atoms and chloride atoms have unfilled orbitals in their outer shells. The lone electron in the outermost shell of a sodium atom can be pulled or knocked out. This ionizes the atom. It is now a positively charged sodium ion. A chlorine atom has an electron vacancy in its outer shell and...
Oxygen transport - methods and oxyhemoglobin
By: HWC, Views: 11563
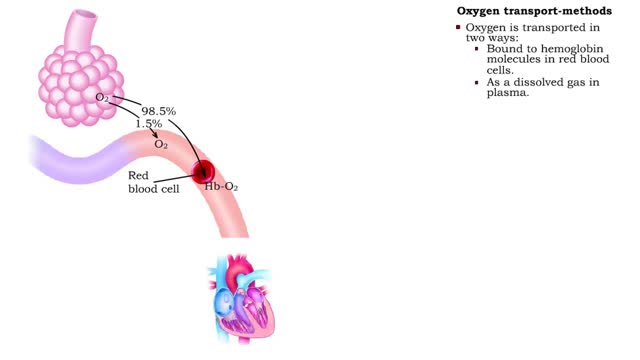
• The blood is the medium used for gas transport throughout the body. • Oxygen is only available in the lungs. Because the partial pressure of oxygen is higher in the alveoli than in the blood, oxygen diffuses into the blood and is transported to systemic cells. • At the tissues the par...
By: HWC, Views: 11226
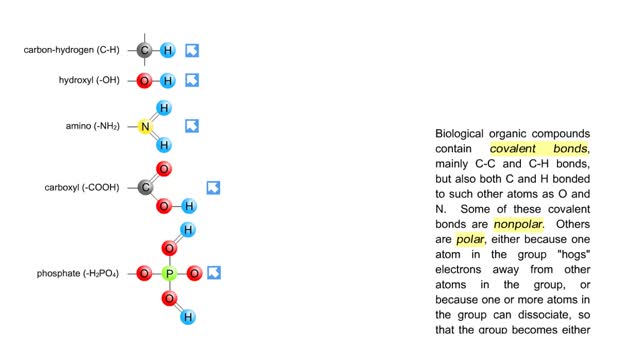
Biological organic compounds contain covalent bonds, mainly C-C and C-H bonds, but also both C and H bonded to such other atoms as O and N. Some of these covalent bonds are nonpolar. Others are polar, either because one atom in the group "hogs" electrons away from other atoms in the group, or...
Resting membrane potential - electrical polarity and maintenance requirements
By: HWC, Views: 11374
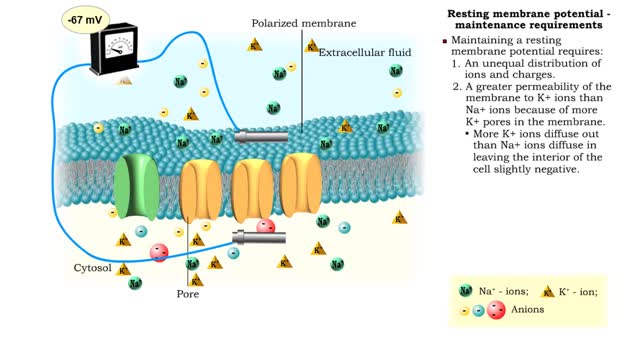
• A resting membrane potential exists when there is a buildup of: 1. positive ions outside the membrane. 2. negative ions inside the membrane. • Membranes with opposing charges are said to be polarized. • The difference in charge applies only to the small distance across the membran...
By: HWC, Views: 10498
The slight positive charge of a hydrogen atom in a water molecule can attract an atom with a slight negative charge, such as the nitrogen in a molecule of ammonia. This forms a hydrogen bond between the two atoms. Hydrogen bonds join the two strands of a DNA molecule. Although hydrogen bo...
Action potentials - electrical characteristics and generation
By: HWC, Views: 11523
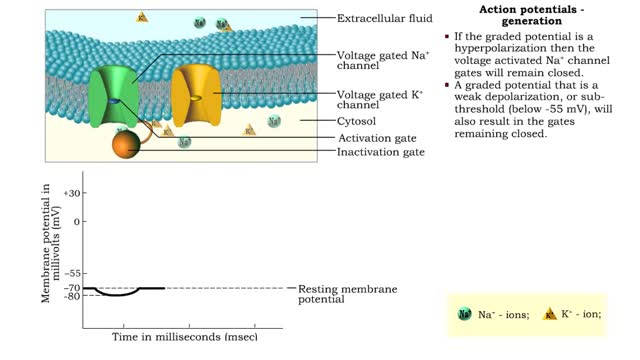
• An action potential is the nervous impulse or signal for long distance communication. Each action potential is generated at the cell's trigger zone. • Action potentials are considered an all-or-nothing phenomena because they are either generated or not. • The generation of an action...
By: Administrator, Views: 14569
Atrophy is the partial or complete wasting away of a part of the body. Causes of atrophy include mutations (which can destroy the gene to build up the organ), poor nourishment, poor circulation, loss of hormonal support, loss of nerve supply to the target organ, excessive amount of apoptosis of c...
Graded potentials - electrical characteristics and types
By: HWC, Views: 11919
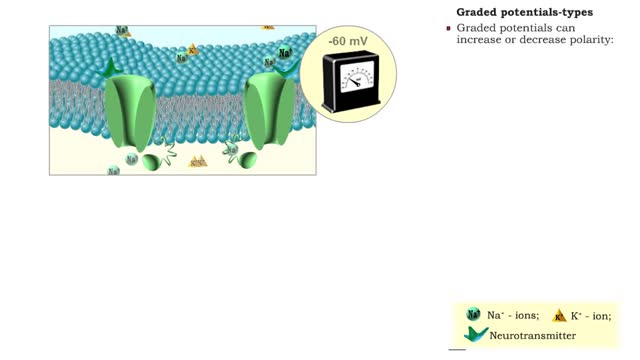
• A graded potential occurs when a gated channel is opened or closed, altering ion flow through the membrane. • Changes in ion and charge distributions cause voltage changes to the resting membrane potential. • The strength of the stimulus determines the number of gated channels affect...
Advertisement