Search Results
Results for: 'SI'
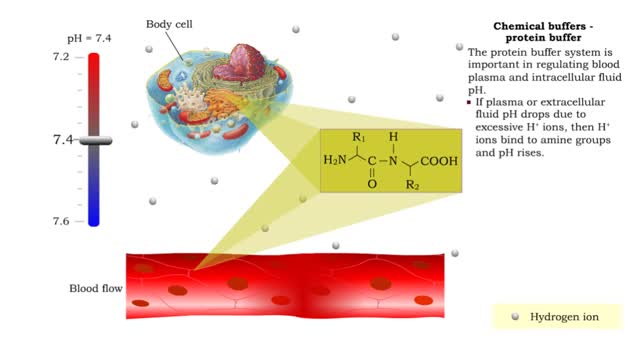
Chemical Buffers - protein buffer, phosphate buffer system and bicarbonate buffer system
By: HWC, Views: 11117
• There are a variety of chemicals in body fluids that prevent the fluids from undergoing large changes in. • These chemicals buffer or regulate fluctuations in H+ concentration. • Chemical buffers: • Bind to H+ ions when there are too many in a solution so pH remains normal. •...
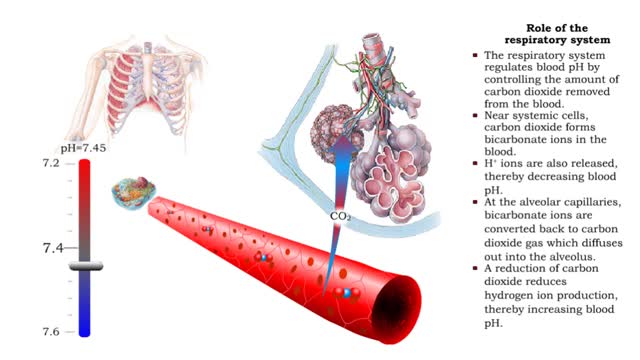
Role of the respiratory system - effect of altered ventilation rates
By: HWC, Views: 11546
• The respiratory system regulates blood pH by controlling the amount of carbon dioxide removed from the blood. • Near systemic cells, carbon dioxide forms bicarbonate ions in the blood. H+ ions are also released, thereby decreasing blood pH. • At the alveolar capillaries, bicarbonate io...
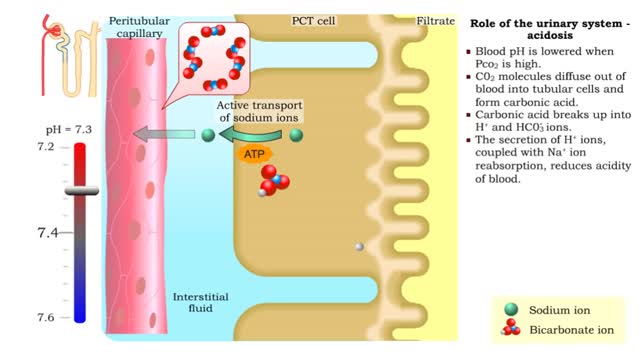
Role of the urinary system - acidosis and alkalosis
By: HWC, Views: 11091
• Tubular cells of the proximal convoluted tubule and collecting tubules can alter filtrate pH and therefore blood pH. • These cells can affect blood pH with two coupled mechanisms: • Reabsorption of bicarbonate ions. • Secretion of hydrogen ions. • The reabsorption of bicarbonate...
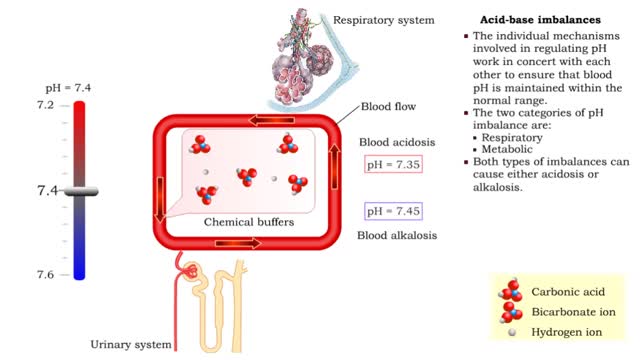
Acid-base imbalances - respiratory acidosis and alkalosis
By: HWC, Views: 11270
• The individual mechanisms involved in regulating pH work in concert with each other to ensure that blood pH is maintained within the normal range. • The two categories of pH imbalance are: • Respiratory • Metabolic • Both types of imbalances can cause either acidosis or alka...
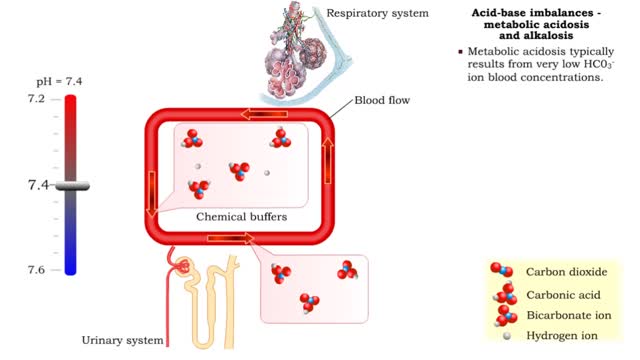
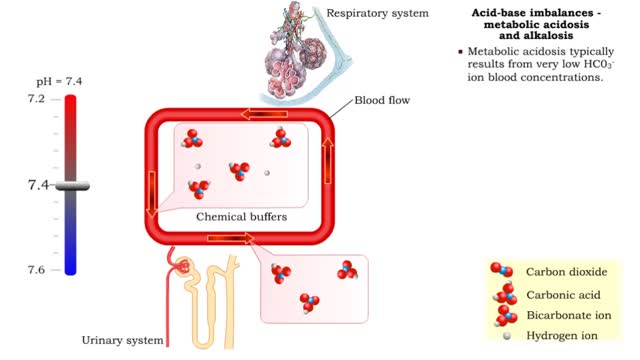
Acid-base imbalances - metabolic acidosis and alkalosis
By: HWC, Views: 11018
• Metabolic acidosis typically results from very low HCO3- ion blood concentrations. • Metabolic alkalosis typically results from very high HCO3- ion blood concentrations.
Acid-base imbalances - compensation of respiratory acidosis and alkalosis
By: HWC, Views: 11174
• When one pH balancing system is affected then the other balancing system attempts to correct, or compensate for, the pH imbalance. - Respiratory acidosis: • Excessive CO2 is present so blood pH becomes acidic. • Compensation is increased secretion of H+ into urine and reabsorption ...
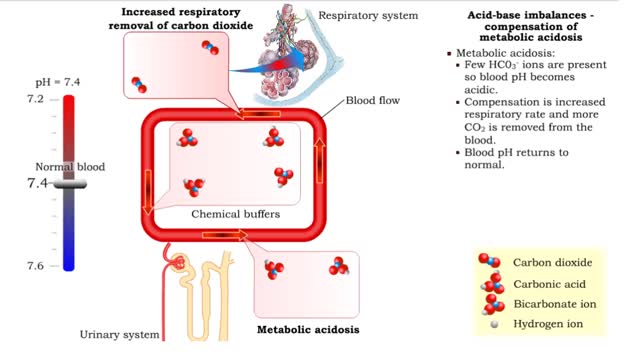
Acid-base imbalances - compensation of metabolic acidosis and alkalosis
By: HWC, Views: 11137
1. Metabolic acidosis: • Few HC03- ions are present so blood pH becomes acidic. • Compensation is increased respiratory rate and more CO2 is removed from the blood. • Blood pH returns to normal. 2. Metabolic alkalosis: • Many HC03- ions are present so blood pH becomes alkaline...
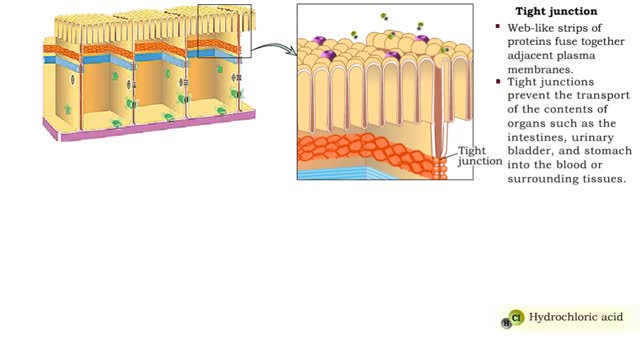
Junction Types - Tight and Adherens Junctions
By: HWC, Views: 11372
Many tissues contain in tercellular junctions between cells. 1. Tight junction 2. Adherens junction 3. Desmosome 4. Hemidesrnosome 5. Gap junction 1. Tight junction • Web-like strips of proteins fuse together adjacent plasma membranes. • Tight junctions prevent the transport...
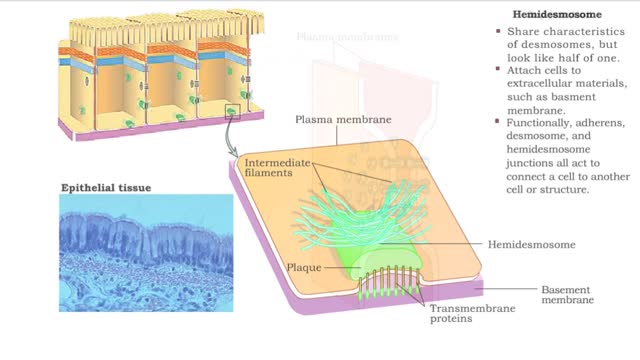
Type of Cell Junctions - Desmosome, Hemidesmosomes and Gap Junctions
By: HWC, Views: 11290
Cell Junctions: Cell junctions are found in some multi-cellular organisms. They exist of complexes and are found between cells and between cells and other structures. The junctions provide a way for cells to connect and exchange signals. What are tight junctions, desmosomes, and gap junctions...
Advertisement