Search Results
Results for: 'SI'
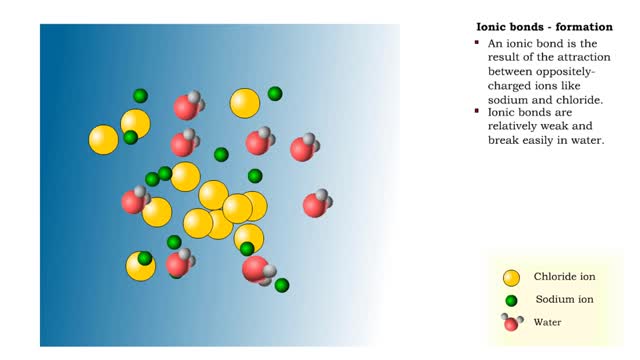
Ionic bonds - role of ions in the body
By: HWC, Views: 11291
Ions • Atoms fill up the outer orbital by transferring electrons from one atom to another. • Atoms now bear a charge and are called ions. • Sodium ion, losing an electron, has a +1 charge. • Chlorine ion, gaining an electron, has a -1 charge. Formation • An ionic bond is t...
Hydrogen bonds - role in the body
By: HWC, Views: 11334
A hydrogen bond is the electromagnetic attraction between polar molecules in which hydrogen is bound to a larger atom, such as oxygen or nitrogen. This is not a sharing of electrons, as in a covalent bond. Instead, this is an attraction between the positive and negative poles of charged atoms. ...
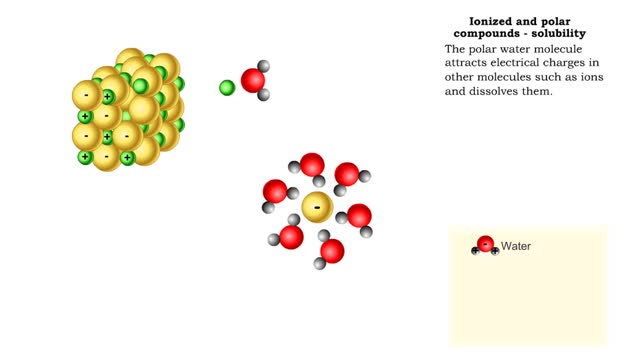
Properties of water -structure of water and polarity (Ionized and polar compounds)
By: HWC, Views: 11171
■ Water transports most of the molecules in the body. ■ The structure of a water molecule allows it to dissolve other molecules. ■ Shared electrons spend more time near the oxygen atom. ■ Oxygen end has a partial negative charge. ■ Hydrogen ends have a partial positive charge....
Non-polar compounds - insolubility
By: HWC, Views: 11138
• A non-polar molecule has uniform distribution of electrons. • Non-polar compounds like fatty acids in lipids have a high proportion of carbon and hydrogen. • Lipids possess no charge or partial charge. • Lipids are not attracted to water molecules. • Lipids are not soluble in...
By: HWC, Views: 10895
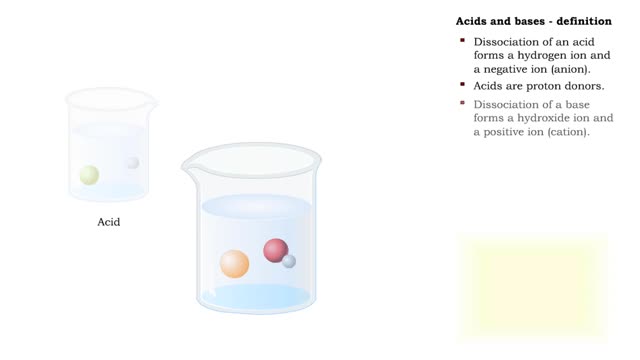
Acids and bases are found all around your house. For example, if you open your pantry or refrigerator, you might see a lot of acids. Fruit juice, soda pop, vinegar, and milk are all examples of acids. The word acid actually comes from a Latin term meaning ''sour.'' Many materials, like sugar for ...
The pH scale - Strong acids and Weak acids
By: HWC, Views: 11092
The pH scale • Expresses concentration of H+. • range: 0-14. • 7 is neutral. • Less 7 is acid. • greater 7 is basic (alkaline). Strong acids - role in the body ■ In strong acids all molecules dissociate. ■ HC1 is highly acidic and found only in the stomach. • H...
Buffers definition and the role of buffer in the body
By: HWC, Views: 11053
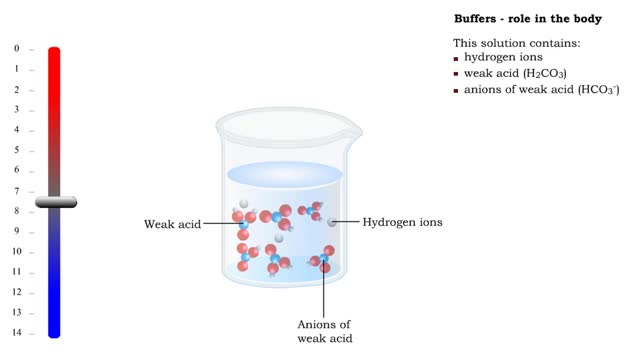
■ Too many H+ break hydrogen bonds and a protein comes apart. ■ Buffers react with excess H+ to protect proteins from breaking down. ■ Buffers consist of weak acid plus anions of that weak acid. This solution contains: • hydrogen ions • weak acid (H2CO3) • anions of we...
Enzyme structure - Properties of enzymes
By: HWC, Views: 10978
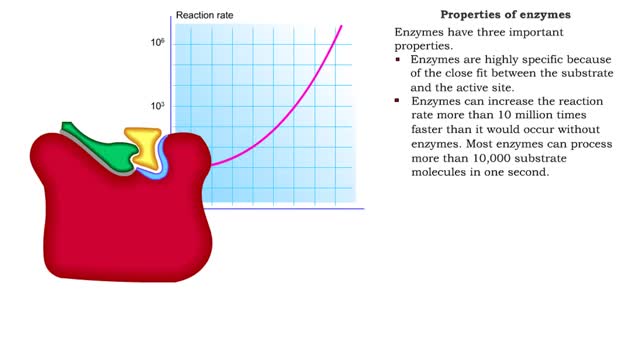
■ Enzymes are proteins that catalyze reactions. ■ Some enzymes have two parts: a protein or apoenzyme and a non-protein or cofactor. ■ Cofactor can be a metal ion or another organic molecule called a coenzyme. ■ Coenzymes often come from vitamins. ■ Cofactors affect the shape of...
By: HWC, Views: 10838
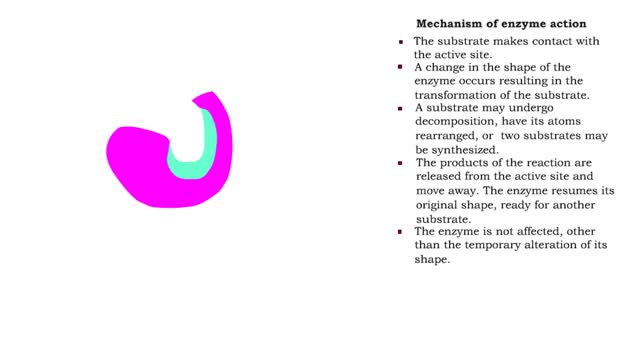
■ The substrate makes contact with the active site. ■ A change in the shape of the enzyme occurs resulting in the transformation of the substrate. ■ A substrate may undergo decomposition, have its atoms rearranged, or two substrates may be synthesized. ■ The products of the reaction...
Advertisement