Search Results
Results for: 'Synthesis of nucleic acids'
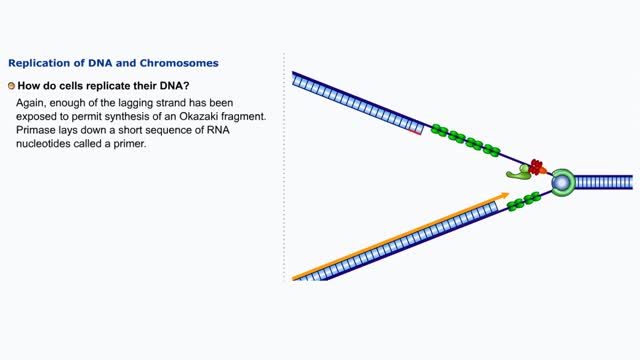
Replication of DNA and Chromosomes/ How do cells replicate their DNA? (Animation) no Audio
By: HWC, Views: 11186
DNA replication in E. coil begins at a site called oriC where a replication bubble forms. At either end of this bubble is a replication fork. Since DNA polymerase Ill can read its DNA template strand only in the 3' to 5' direction this means that one strand (leading) can be read continuously b...
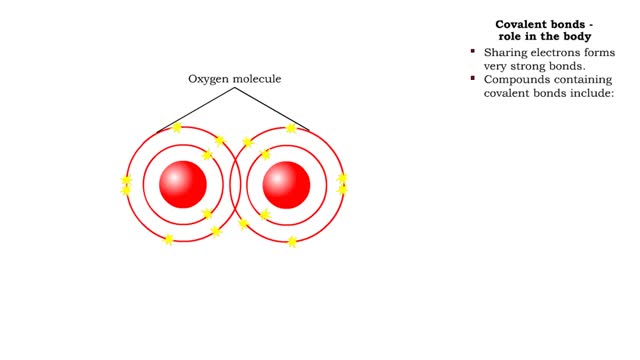
Covalent bonds - role in the body
By: HWC, Views: 11314
A covalent bond is formed when atoms share one or more pairs of electrons. This is opposed to an ionic bond, where electrons are actually transferred from one atom to another. Formation • Atoms fill up the outer orbital by sharing electrons. • Two oxygen atoms sharing electrons form on...
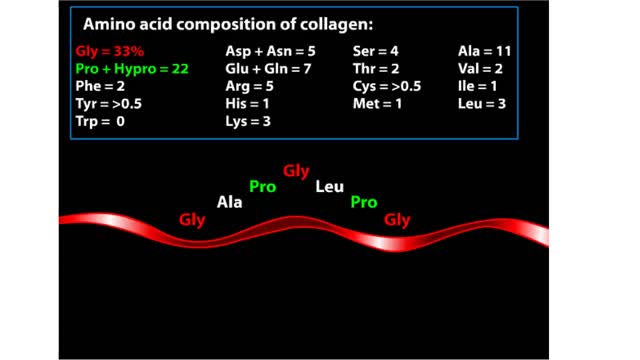
Major Elements in Biological Molecules: Proteins
By: HWC, Views: 10832
Proteins are chains of amino acids linked by peptide bonds. The 20 different amino acids used to make all proteins differ only in their side chains, and the properties of these side chains account for the great diversity of protein structure and function. Collagen is an example of how a prote...
By: HWC, Views: 10337
Living things must accomplish a great number of tasks just to get through a day, and these tasks are accomplished by a diverse range of biological molecules. In the range of tasks that molecules accomplish, however, proteins reign supreme. Almost every chemical reaction that takes place in living...
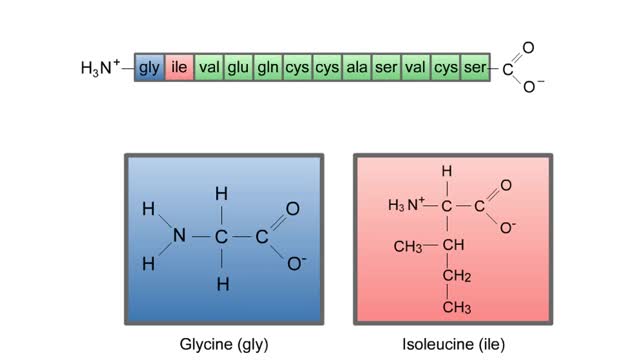
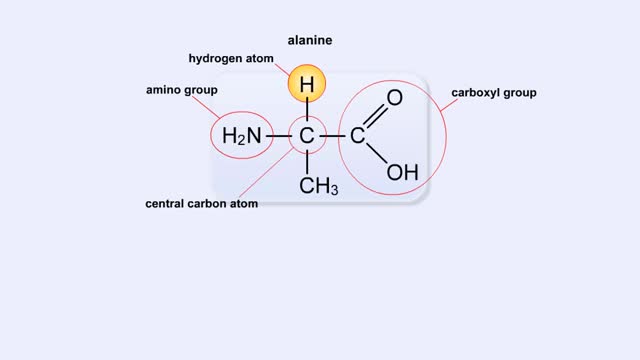
Structure of Amino Acid, Peptide Bonds & Polypeptides
By: HWC, Views: 10920
Here are the molecular formulas of three different amino acids. All amino acids share this backbone. The main difference between every amino acid is the side groups seen here, and these side groups give each of the amino acids their different characteristics. But before we get into that, let's ...
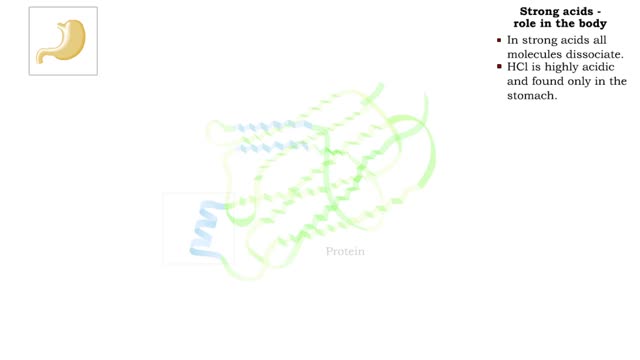
The pH scale - Strong acids and Weak acids
By: HWC, Views: 11420
The pH scale • Expresses concentration of H+. • range: 0-14. • 7 is neutral. • Less 7 is acid. • greater 7 is basic (alkaline). Strong acids - role in the body ■ In strong acids all molecules dissociate. ■ HC1 is highly acidic and found only in the stomach. • H...
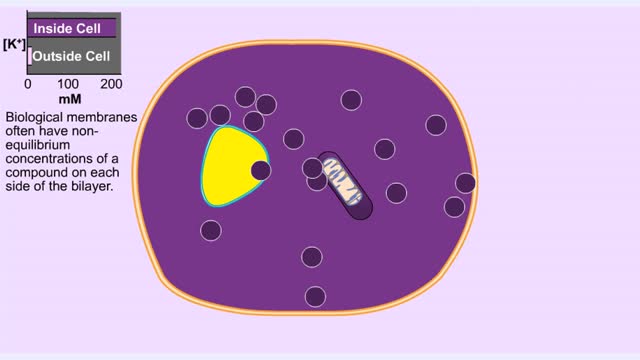
Membrane Protein and Facilitated Transport (Passive Vs Active)
By: HWC, Views: 10949
Membrane proteins are common proteins that are part of, or interact with, biological membranes. Membrane proteins fall into several broad categories depending on their location. Integral membrane proteins span the membrane, with hydrophobic amino acids interacting with the lipid bilayer and hy...
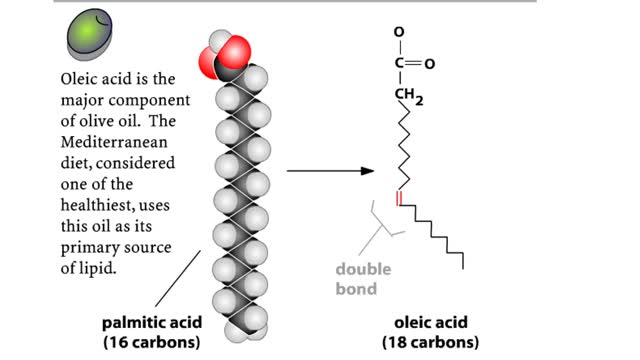
Major Elements in Biological Molecules: Lipids
By: HWC, Views: 10717
A triglyceride (also called triacylglycerol) is composed of three fatty acid molecules and one glycerol molecule. The fatty acids attach to the glycerol molecule by a covalent ester bond. The long hydrocarbon chain of each fatty acid makes the triglyceride molecule nonpolar and hydrophobic. Pa...
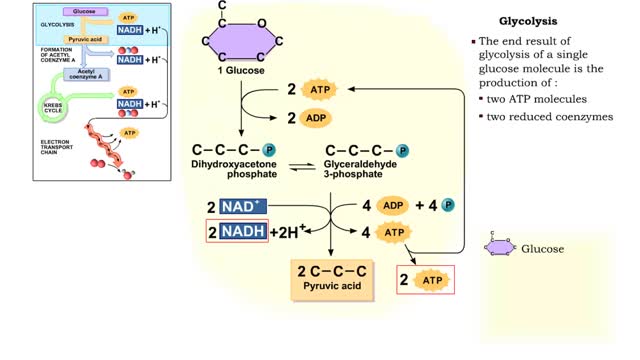
By: HWC, Views: 11637
The first reactions involve a single 6-carbon glucose sugar undergoing phosphorylation using two ATP molecules and resulting in two 3-carbon compounds. • The rest of this pathway involves an oxidation reduction reaction, forming two reduced coenzymes, and generation of four ATP molecules. ...
Advertisement