Search Results
Results for: 'deoxyribose nucleic acid'
Digestive chemicals - water, gastric acid, bile & bicarbonate
By: HWC, Views: 10789
• Water is the most abundant molecule in ingested fluids. • Water plays a primary role in hydrolytic digestive reactions. • Helps liquefy and transport digestive foodstuffs down the tract. • Transports secretions from accessory digestive organs to gastrointestinal tract. • Aids ...
By: HWC, Views: 10901
Acids and bases are found all around your house. For example, if you open your pantry or refrigerator, you might see a lot of acids. Fruit juice, soda pop, vinegar, and milk are all examples of acids. The word acid actually comes from a Latin term meaning ''sour.'' Many materials, like sugar for ...
Proteins Defined, Hierarchy & Composition of Cells
By: HWC, Views: 10521
Proteins are long chains of amino acids linked together by peptide bonds. Together with the other three biological macromolecules—carbohydrates, lipids, and nucleic acids—proteins are the building blocks of cells. Proteins are the most complex and abundant biological macromolecules in cel...
By: HWC, Views: 11229
• The amount of ATP stored in a skeletal muscle cell can only provide muscular activity for two to three seconds. • Muscle cells must be able to generate additional molecules of ATP to continue contracting. • Muscle cells can generate ATP from several processes: • Phosphogen syste...
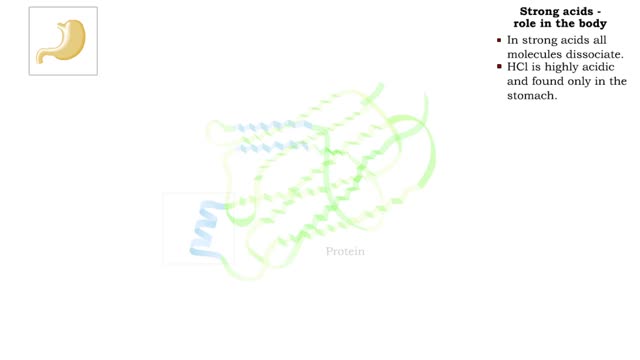
The pH scale - Strong acids and Weak acids
By: HWC, Views: 11098
The pH scale • Expresses concentration of H+. • range: 0-14. • 7 is neutral. • Less 7 is acid. • greater 7 is basic (alkaline). Strong acids - role in the body ■ In strong acids all molecules dissociate. ■ HC1 is highly acidic and found only in the stomach. • H...
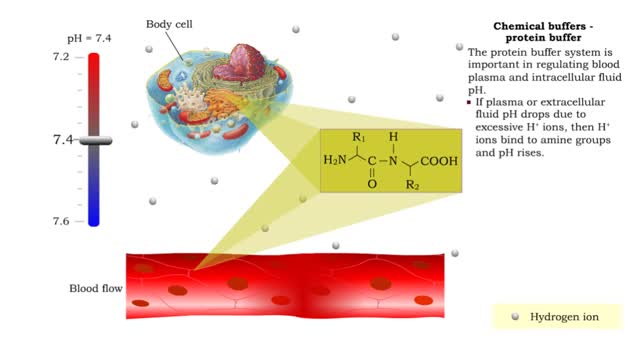
Chemical Buffers - protein buffer, phosphate buffer system and bicarbonate buffer system
By: HWC, Views: 11207
• There are a variety of chemicals in body fluids that prevent the fluids from undergoing large changes in. • These chemicals buffer or regulate fluctuations in H+ concentration. • Chemical buffers: • Bind to H+ ions when there are too many in a solution so pH remains normal. •...
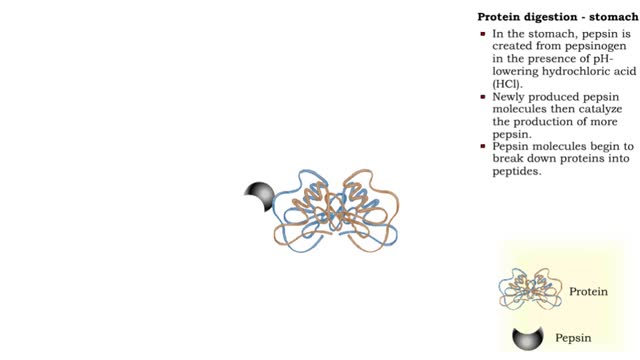
Protein digestion - stomach & small intestine
By: HWC, Views: 10533
• Protein digestion occurs in the stomach and small intestine. • The stomach enzyme pepsin initiates the process. • Pancreatic and intestinal brush border enzymes complete the digestive process. • In the stomach, pepsin is created from pepsinogen in the presence of pH-lowering hyd...
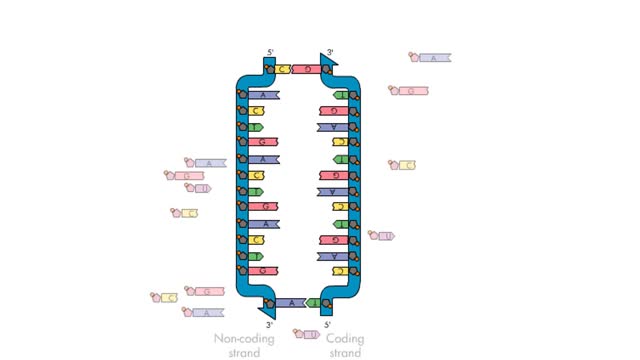
Transcription—A molecular view
By: HWC, Views: 6692
Transcription, as related to genomics, is the process of making an RNA copy of a gene's DNA sequence. This copy, called messenger RNA (mRNA), carries the gene's protein information encoded in DNA. During transcription, a DNA molecule is copied into RNA molecules that are then used to translate...
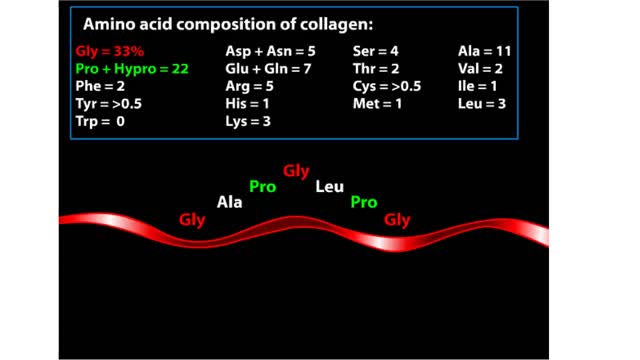
Major Elements in Biological Molecules: Proteins
By: HWC, Views: 10542
Proteins are chains of amino acids linked by peptide bonds. The 20 different amino acids used to make all proteins differ only in their side chains, and the properties of these side chains account for the great diversity of protein structure and function. Collagen is an example of how a prote...
Advertisement