Search Results
Results for: 'hydrogen ion concentration'
By: HWC, Views: 4782
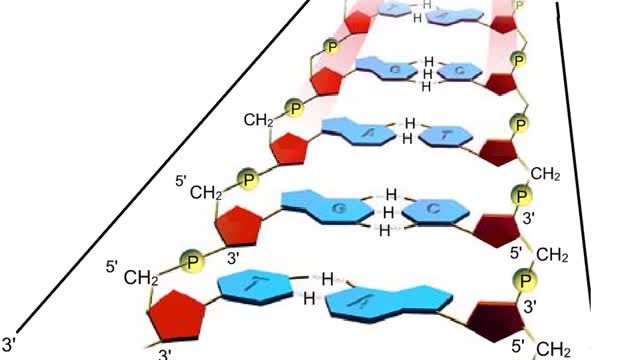
A section from a DNA double helix The backbone of each DNA strand consists of alternating deoxyribose sugars and phosphate groups. The two strands run in opposite directions. One runs from the 5' to 3' direction, the other in the 3' to 5' direction. Think of the deoxyribose units o...
Michaelis–Menten equation & Kinetic parameters
By: HWC, Views: 10737
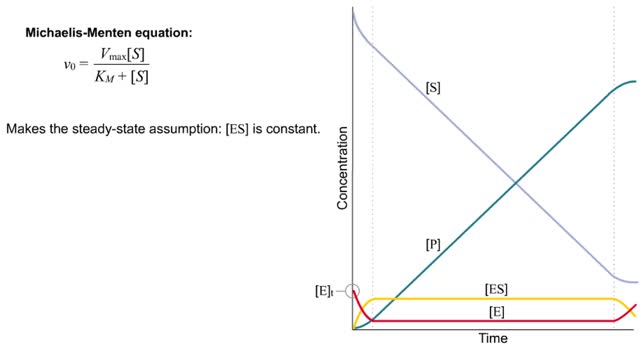
The Michaelis–Menten equation is the rate equation for a one-substrate enzyme-catalyzed reaction. This equation relates the initial reaction rate (v0), the maximum reaction rate (Vmax), and the initial substrate concentration [S] through the Michaelis constant KM—a measure of the substrat...
What are Strong & Weak Acids and How they're different?
By: HWC, Views: 9696
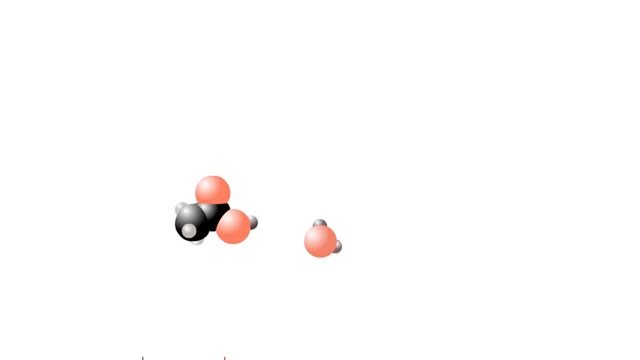
Let's consider the changes that take place when hydrogen chloride, HCI, is added to water. You will need to recognize space-filling models of HCI molecules, hydronium ions (H30+), chloride ions (C11, and water molecules (H20). They are shown at the right. When HC1 molecules dissolve in water, ...
Facilitated Diffusion - Glucose transport
By: HWC, Views: 11175
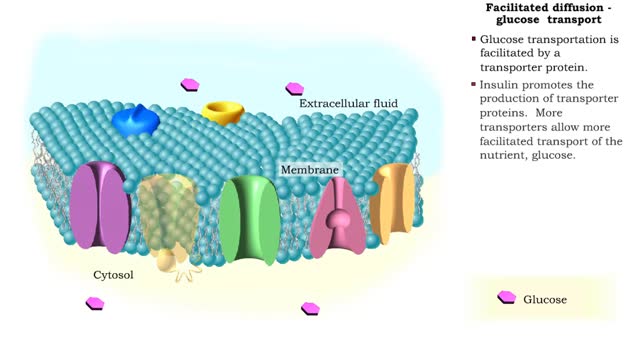
Transmembrane proteins help solutes that are too polar or too highly charged move through the lipid bilayer The processes involved are: Channel mediated facilitated diffusion Carrier mediated facilitated diffusion In facilitated diffusion, molecules only move with the aid of a protein i...
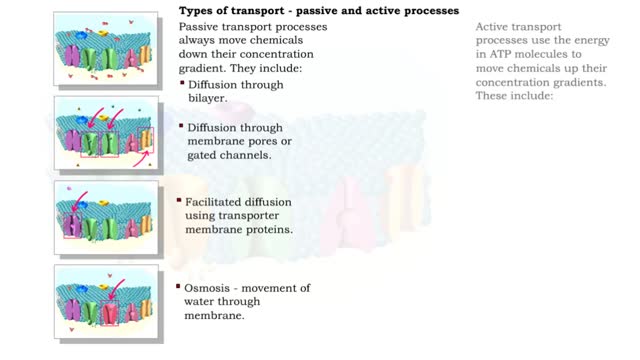
Type of Transport - Active and Passive Processes
By: HWC, Views: 11232
Active transport moves materials from lower to a higher concentration, while passive transport moves materials from higher to lower concentration. Active transport requires energy to proceed, while passive transport does not require the input of extra energy to occur. Transport processes that ...
By: HWC, Views: 5118
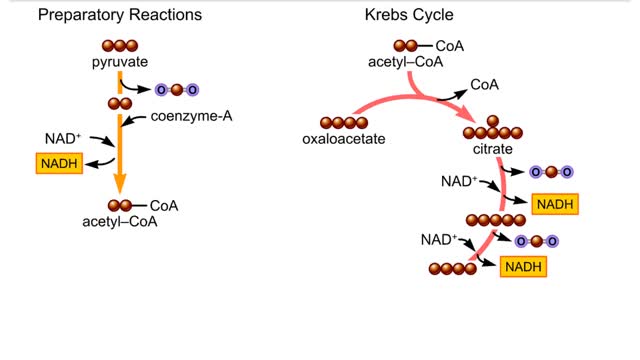
The second-stage reactions of aerobic respiration. The second-stage reactions occur in a mitochondrion's inner compartment. In the first preparatory reaction, a carbon atom is stripped from pyruvate and released as carbon dioxide. The remaining carbons combine with coenzyme A and give ...
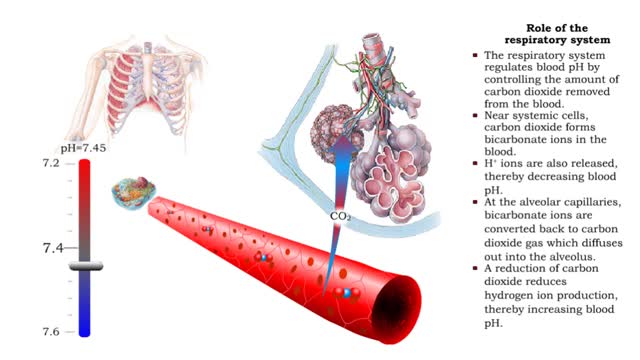
Role of the respiratory system - effect of altered ventilation rates
By: HWC, Views: 11508
• The respiratory system regulates blood pH by controlling the amount of carbon dioxide removed from the blood. • Near systemic cells, carbon dioxide forms bicarbonate ions in the blood. H+ ions are also released, thereby decreasing blood pH. • At the alveolar capillaries, bicarbonate io...
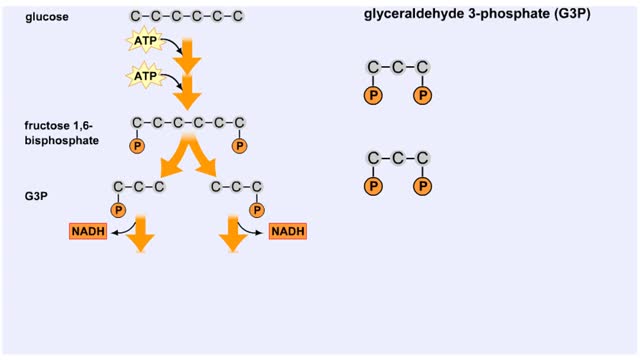
Splitting of Sugar, Oxidation/ Reduction & ATP Generation
By: HWC, Views: 10661
The next reaction shows us the meaning of "glycolysis" or the splitting of glucose. The fructose bisphosphate molecule is split into two molecules each containing 3 carbons as the backbone. FBP is split into two 3-carbon molecules called G3P, or glyceraldehyde 3-phosphate. Notice that the phos...
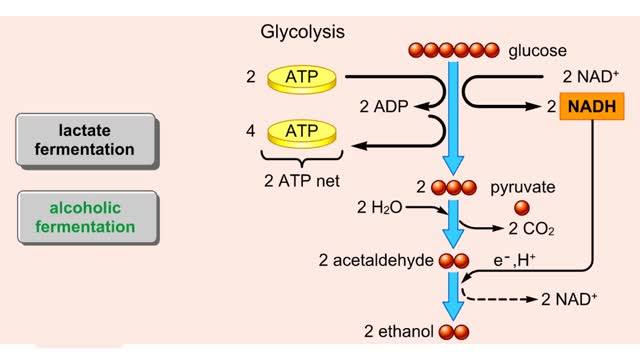
By: HWC, Views: 4803
Both lactate fermentation and alcoholic fermentation begin with pyruvate formed by glycolysis. During lactate fermentation, pyruvate molecules accept electrons and hydrogen from NADH. This transfer regenerates NAD± and converts pyruvate to lactate. The net energy yield is the two ATP...
Advertisement