Search Results
Results for: 'hydrogen ion concentration'
Bond in biological molecules (Ionic, Covalent and Hydrogen bonds)/ How atoms bond?
By: HWC, Views: 8232
Sodium atoms and chloride atoms have unfilled orbitals in their outer shells. The lone electron in the outermost shell of a sodium atom can be pulled or knocked out. This ionizes the atom. It is now a positively charged sodium ion. A chlorine atom has an electron vacancy in its outer shell and...
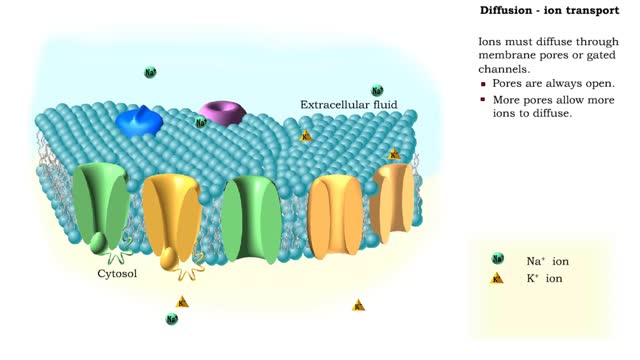
Simple Diffusion - Ion transport
By: HWC, Views: 10947
In the process of diffusion, a substance tends to move from an area of high concentration to an area of low concentration until its concentration becomes equal throughout a space. Ions must diffuse through membrane pores or gated channels. Pores are always open. More pores allow more ions...
By: HWC, Views: 8078
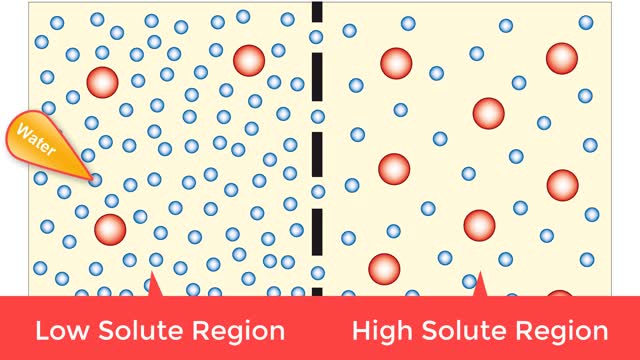
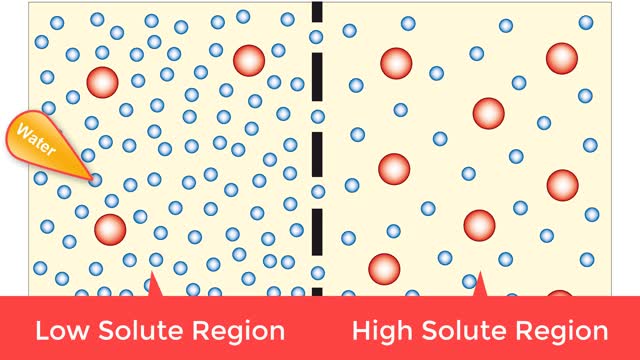
Osmosis is when a solvent, such as water, moves from a low-solute concentration solution to a higher-solute concentration solution through a semipermeable. Osmosis is an example of diffusion (a special case of diffusion) in which the molecules are water, and the concentration gradient occurs a...
By: HWC, Views: 8577
Osmosis is when a solvent, such as water, moves from a low-solute concentration solution to a higher-solute concentration solution through a semipermeable. Osmosis is an example of diffusion (a special case of diffusion) in which the molecules are water, and the concentration gradient occurs a...
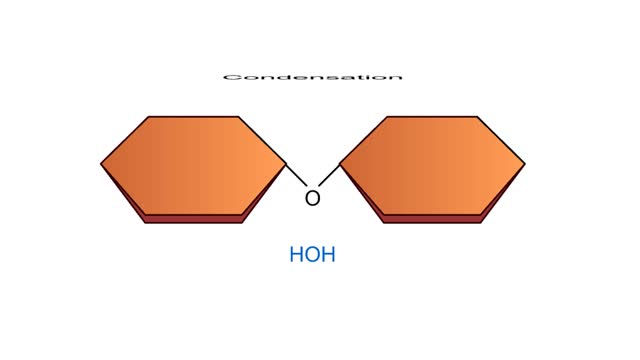
Condensation and Hydrolysis Animation
By: HWC, Views: 4719
A condensation reaction joins two molecules together to form one larger molecule. An enzyme removes a hydroxyl group from one molecule and a hydrogen atom from another, then speeds the formation of a bond between the two molecules at their exposed sites. Typically the discarded atoms join t...
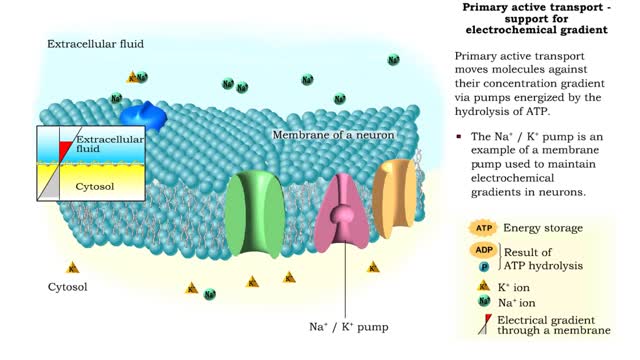
Primary Active Transport - electrochemical gradient and ion transport / water movement
By: HWC, Views: 11084
Energy derived from ATP changes the shape of a transporter protein which pumps a substance across a plasma membrane against its concentration gradient An electrochemical gradient is a gradient of electrochemical potential, usually for an ion that can move across a membrane. The gradient consis...
By: HWC, Views: 10785
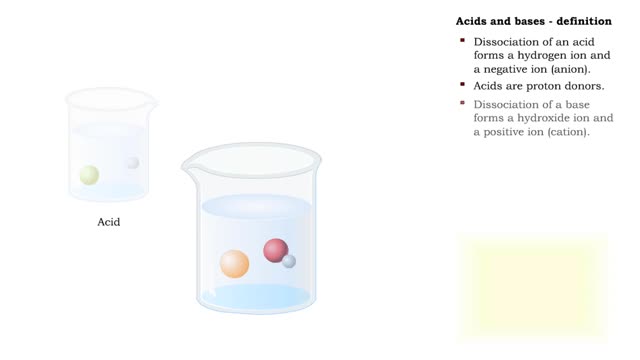
Acids and bases are found all around your house. For example, if you open your pantry or refrigerator, you might see a lot of acids. Fruit juice, soda pop, vinegar, and milk are all examples of acids. The word acid actually comes from a Latin term meaning ''sour.'' Many materials, like sugar for ...
Major Elements in Biological Molecules: Nucleic acids
By: HWC, Views: 10861
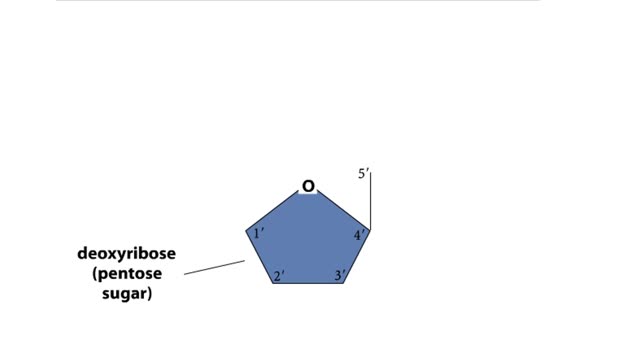
DNA and RNA are nucleic acids (polymers of nucleotides). Two polymers with complementary nucleotide sequences can pair with each other. This pairing endows nucleic acids with the ability to store, transmit, and retrieve genetic information. Two strands of DNA pair by hydrogen bonding. A compon...
Secondary and tertiary levels of protein structure Animation
By: HWC, Views: 4782
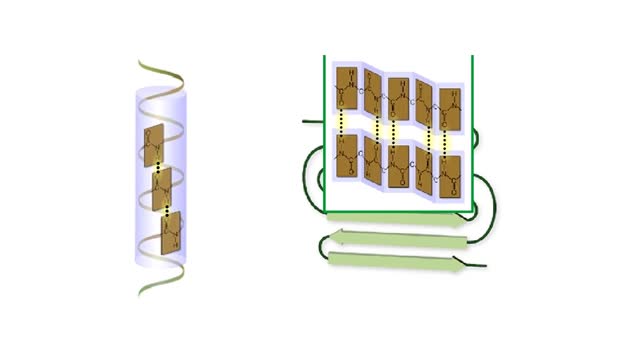
Amino acid sequence dictates a protein's final shape. The presence of certain amino acids favors a pattern of hydrogen bonding that causes part of the polypeptide chain to coil and twist into an alpha helix. The presence of other amino acids enables hydrogen bonding between strand like re...
Advertisement