Search Results
Results for: 'hydrogen ion concentration'
By: HWC, Views: 11405
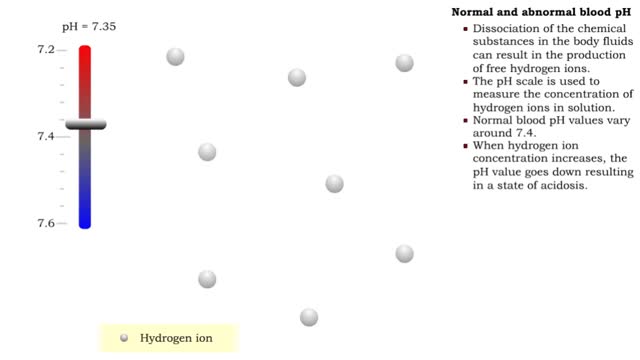
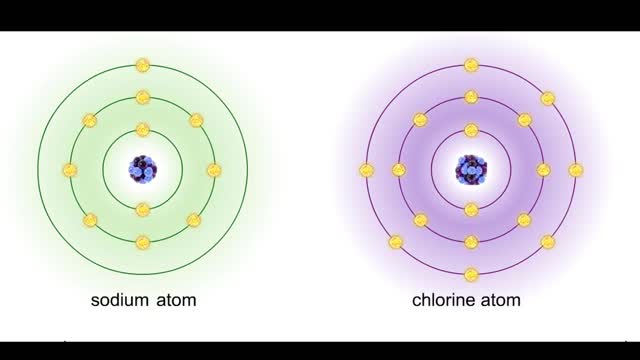
• Dissociation of the chemical substances in the body fluids can result in the production of free hydrogen ions. • The pH scale is used to measure the concentration of hydrogen ions in solution. • Normal blood pH values vary around 7.4. • When hydrogen ion concentration increases, t...
Buffers definition and the role of buffer in the body
By: HWC, Views: 11357
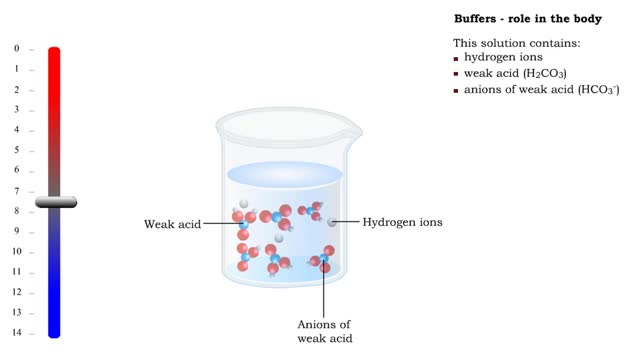
■ Too many H+ break hydrogen bonds and a protein comes apart. ■ Buffers react with excess H+ to protect proteins from breaking down. ■ Buffers consist of weak acid plus anions of that weak acid. This solution contains: • hydrogen ions • weak acid (H2CO3) • anions of we...
Hydrogen bonds - role in the body
By: HWC, Views: 11634
A hydrogen bond is the electromagnetic attraction between polar molecules in which hydrogen is bound to a larger atom, such as oxygen or nitrogen. This is not a sharing of electrons, as in a covalent bond. Instead, this is an attraction between the positive and negative poles of charged atoms. ...
By: HWC, Views: 11938
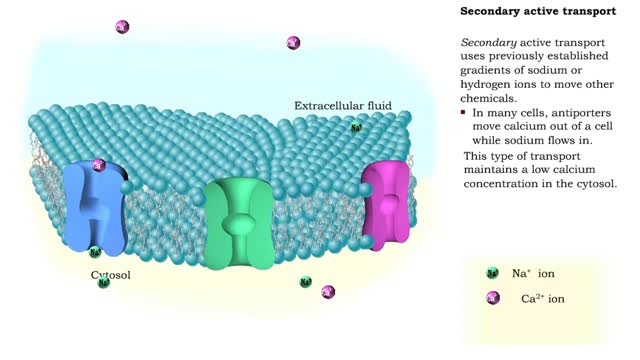
Energy stored (in a hydrogen or sodium concentration gradient) is used to drive other substances against their own concentration gradients Secondary active transport, is transport of molecules across the cell membrane utilizing energy in other forms than ATP. In many cells, antiporters mov...
Role of the respiratory system - effect of altered ventilation rates
By: HWC, Views: 11230
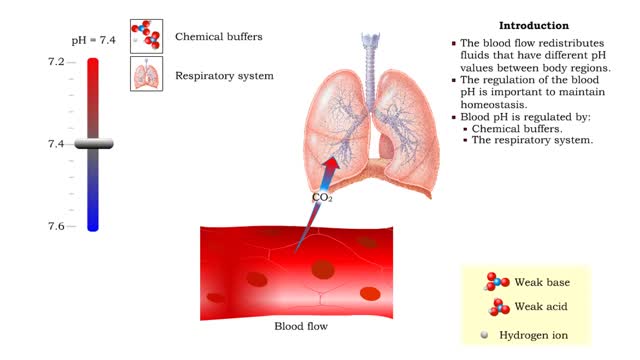
• Dissociation of the chemical substances in the body fluids can result in the production of free hydrogen ions. • The pH scale is used to measure the concentration of hydrogen ions in solution. • Normal blood pH values vary around 7.4. • When hydrogen ion concentration increases, t...
By: HWC, Views: 10115
The slight positive charge of a hydrogen atom in a water molecule can attract an atom with a slight negative charge, such as the nitrogen in a molecule of ammonia. This forms a hydrogen bond between the two atoms. Hydrogen bonds join the two strands of a DNA molecule. Although hydrogen bo...
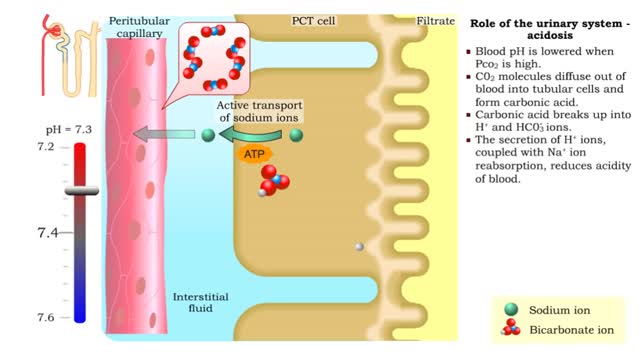
Role of the urinary system - acidosis and alkalosis
By: HWC, Views: 11489
• Tubular cells of the proximal convoluted tubule and collecting tubules can alter filtrate pH and therefore blood pH. • These cells can affect blood pH with two coupled mechanisms: • Reabsorption of bicarbonate ions. • Secretion of hydrogen ions. • The reabsorption of bicarbonate...
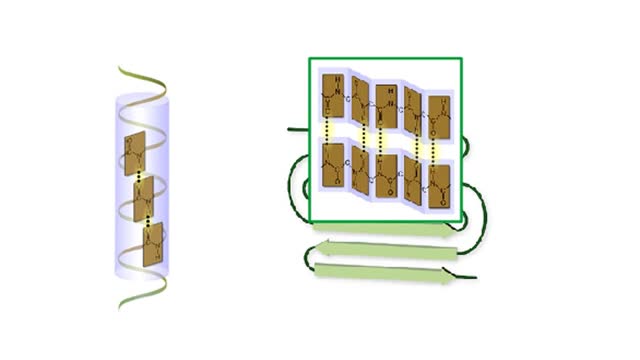
Protein Secondary and Tertiary Structures - Animation
By: HWC, Views: 7530
Amino acid sequence dictates a protein's final shape. The presence of certain amino acids favors a pattern of hydrogen bonding that causes part of the polypeptide chain to coil and twist into an alpha helix. The presence of other amino acids enables hydrogen bonding between strand like r...
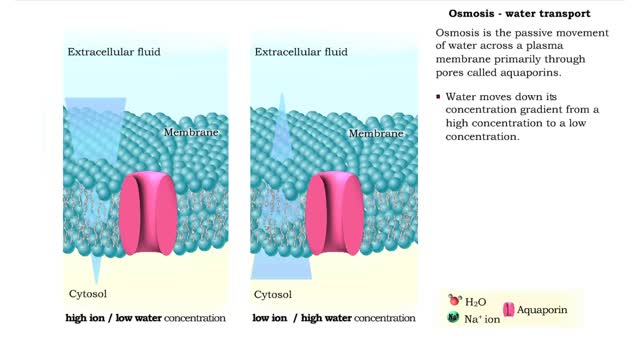
By: HWC, Views: 11487
Osmosis is the flow of water down its concentration gradient, across a semi-permeable membrane. Osmosis is an example of diffusion, which is when molecules tend to distribute themselves evenly in a space. what is a semi-permeable membrane? It is a membrane or barrier that allows some molec...
Advertisement