Search Results
Results for: 'Facilitated diffusion'
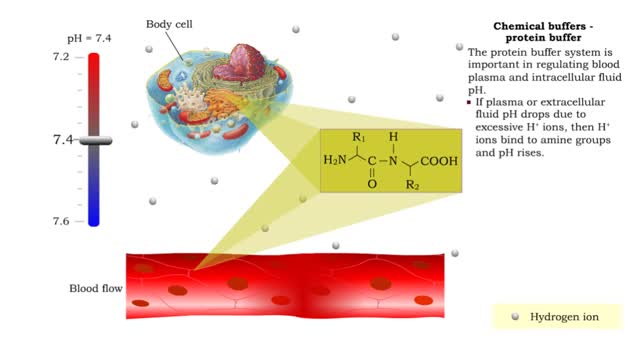
Chemical Buffers - protein buffer, phosphate buffer system and bicarbonate buffer system
By: HWC, Views: 11206
• There are a variety of chemicals in body fluids that prevent the fluids from undergoing large changes in. • These chemicals buffer or regulate fluctuations in H+ concentration. • Chemical buffers: • Bind to H+ ions when there are too many in a solution so pH remains normal. •...
By: HWC, Views: 8205
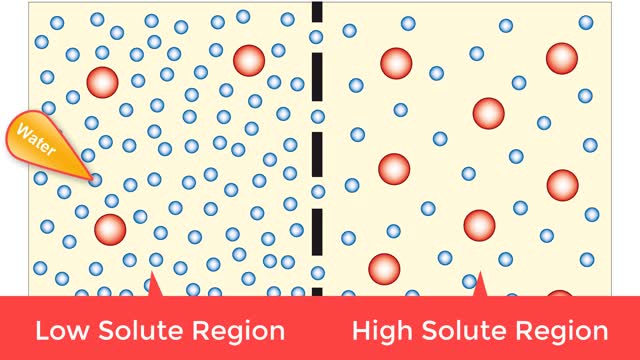
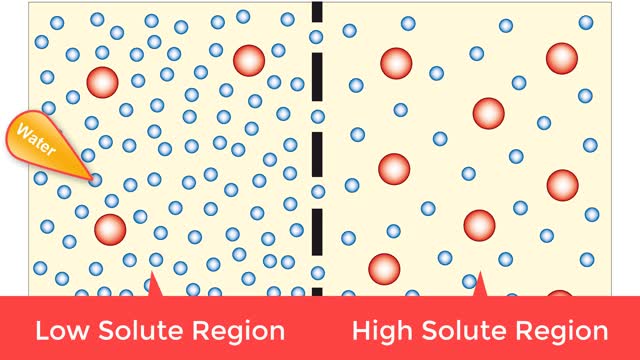
Osmosis is when a solvent, such as water, moves from a low-solute concentration solution to a higher-solute concentration solution through a semipermeable. Osmosis is an example of diffusion (a special case of diffusion) in which the molecules are water, and the concentration gradient occurs a...
By: HWC, Views: 8704
Osmosis is when a solvent, such as water, moves from a low-solute concentration solution to a higher-solute concentration solution through a semipermeable. Osmosis is an example of diffusion (a special case of diffusion) in which the molecules are water, and the concentration gradient occurs a...
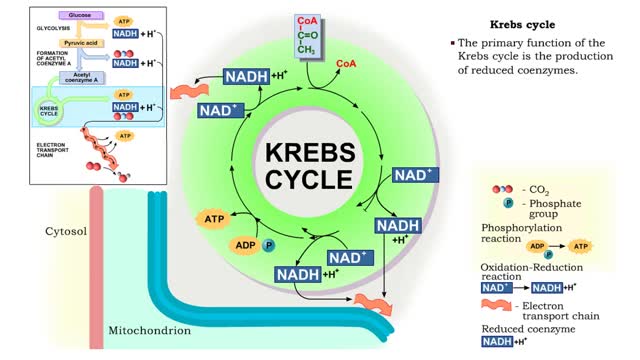
Krebs cycle : Formation of acetyl coenzyme A and Electron transport chain
By: HWC, Views: 11236
The oxidation of glucose to produce ATP is cellular respiration. Four sets of reactions are involved: Glycolysis Formation of acetyl coenzyme A Krebs cycle reactions Electron transport chain reactions • The second pathway of glucose catabolism, formation of acetyl coenzyme A, is a transi...
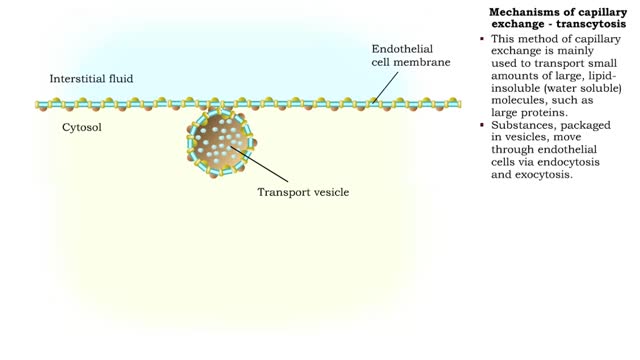
Mechanisms of capillary exchange
By: HWC, Views: 11223
■ The primary role of capillaries is to permit the exchange of nutrients and wastes between the blood and tissue cells (via interstitial fluid). ■ Oxygen and nutrients move from the blood to the cells. ■ Carbon dioxide and other wastes move from the cells to the blood. The three ba...
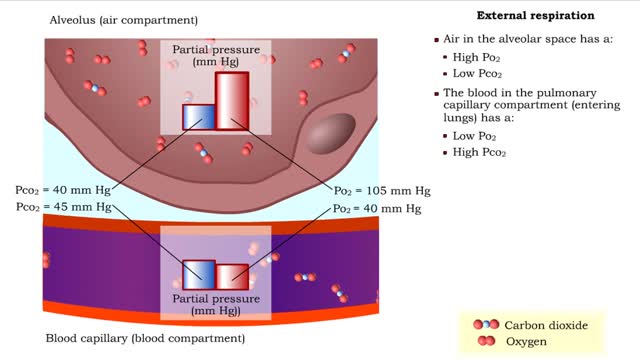
Gas exchange - partial pressure, locations, external and internal respiration
By: HWC, Views: 11197
▪ In a mixture, each individual gas exerts a pressure that is proportional to the concentration of that gas within the mixture. • This part of the total pressure is called a "partial pressure". • A gas moves along the part of the pressure gradient determined by its own concentration. ...
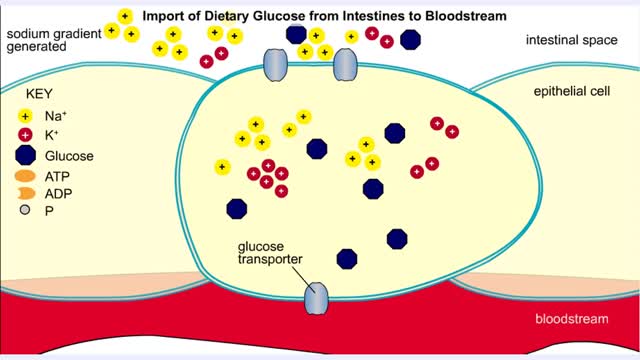
Import of Dietary Glucose from Intestines to Bloodstream
By: HWC, Views: 10443
• Membranes have hydrophobic interiors. which resist the passage of hydrophilic compounds and ions. • However. transporter membrane proteins facilitate the passage of these molecules. • Passive transporters accelerate diffusion of molecules towards equilibrium (decrease a concentrat...
Molecules, Membrane Permeability and Structure
By: HWC, Views: 10488
Organisms are not isolated system at equilibrium and need to intake nutrients and electrolytes as remove wastes. Similarly Cells within an organism must also exchange compound by passing them through membrane. The permeability of a membrane is the rate of passive diffusion of molecules th...
By: HWC, Views: 10184
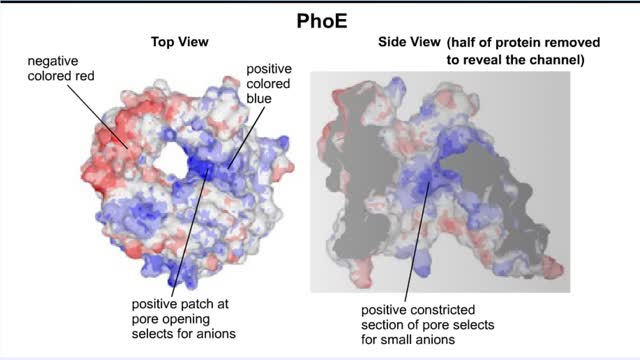
Transmembrane channels, also called membrane channels, are pores within a lipid bilayer. The channels can be formed by protein complexes that run across the membrane or by peptides. They may cross the cell membrane, connecting the cytosol, or cytoplasm, to the extracellular matrix. Membrane po...
Advertisement