Search Results
Results for: 'lipid-soluble molecules'
HIV replication/ Replication cycle of HIV
By: HWC, Views: 8909
Replication cycle of HIV, one of the retroviruses. The HIV virus is surrounded by a lipid envelope with embedded proteins. A coat of viral proteins surrounds two strands of RNA and the enzymes used during replication. The virus attaches to and enters the host cell. Viral reverse trans...
By: HWC, Views: 11331
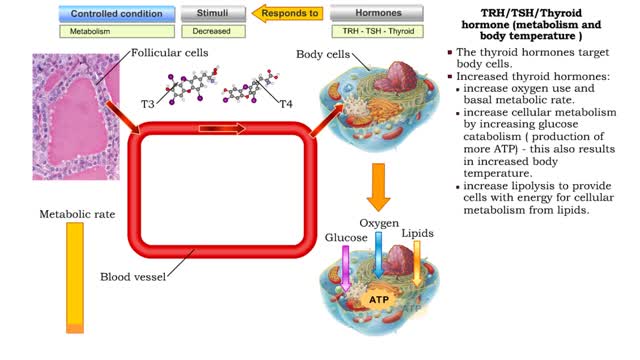
Thyroid hormone production • A decline in metabolic rate caused by increased metabolic need or physical exertion stimulates the production of thyrotropin hormone releasing (TRH) hormone from the cells of the hypothalamus. • Thyrotropin hormone releasing hormone targets the thyrotrophic ce...
By: HWC, Views: 11553
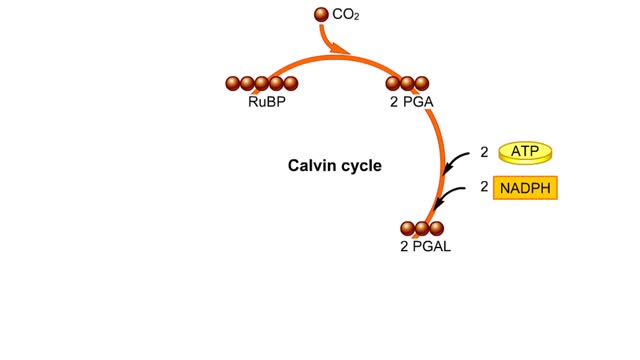
he light-independent reactions make sugars by way of a cyclic pathway called the Calvin cycle. The cycle begins when rubisco attaches a carbon from carbon dioxide to ribulose bisphosphate. The molecule that forms splits into two molecules of PGA. Each PGA gets a phosphate group from ATP a...
Oxygen transport: association and dissociation & Factors that affect hemoglobin's saturation with O2
By: HWC, Views: 11613
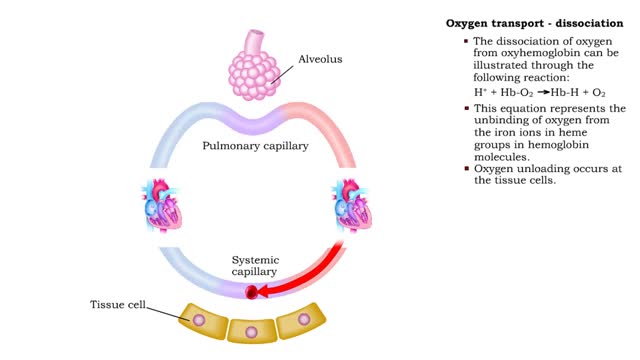
• The production of oxyhemoglobin can be illustrated through the following reaction: 02 + Hb-H --) Hb-02 + H+ • This equation represents the binding of oxygen to the iron ions in heme groups in hemoglobin molecules. • Oxygen binding or loading occurs at the lungs • The dissociatio...
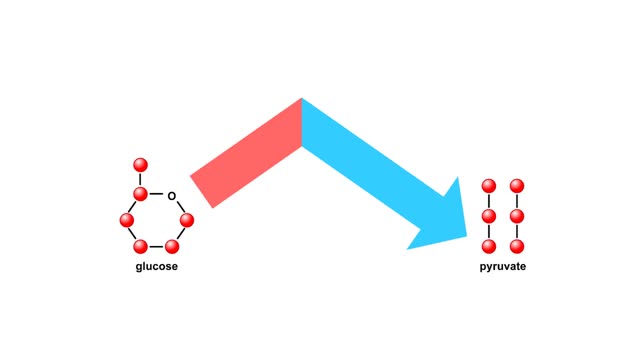
Energy inputs and release in glycolysis Animation
By: HWC, Views: 5601
Glycolysis breaks the six-carbon sugar glucose into two three-carbon molecules of pyruvate. The first steps of glycolysis require an energy input in the form of two phosphate-group transfers from ATP. These phosphorylations raise the energy level of glucose enough to allow the energy-releas...
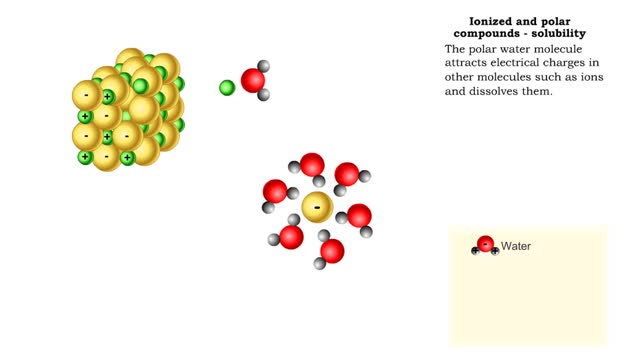
Properties of water -structure of water and polarity (Ionized and polar compounds)
By: HWC, Views: 11871
■ Water transports most of the molecules in the body. ■ The structure of a water molecule allows it to dissolve other molecules. ■ Shared electrons spend more time near the oxygen atom. ■ Oxygen end has a partial negative charge. ■ Hydrogen ends have a partial positive charge....
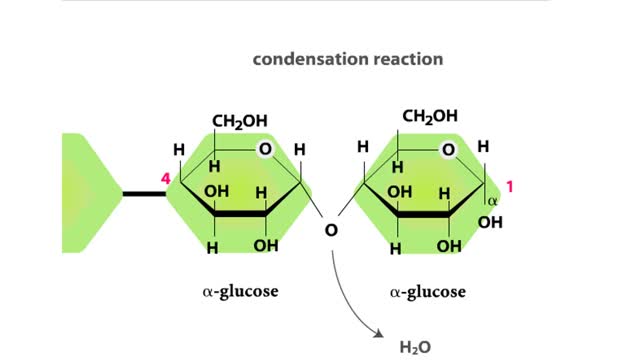
Major Elements in Biological Molecules: Carbohydrates
By: HWC, Views: 11281
Carbohydrates include simple sugars (monosaccharides) as well as large polymers (polysaccharides). Glucose is a hexose, a sugar composed of six carbon atoms, usually found in ring form. A starch macromolecule is a polysaccharide composed of thousands of glucose units. Glucose molecules can be ...
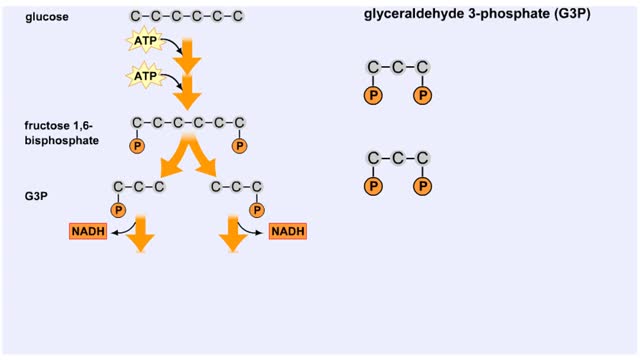
Splitting of Sugar, Oxidation/ Reduction & ATP Generation
By: HWC, Views: 11440
The next reaction shows us the meaning of "glycolysis" or the splitting of glucose. The fructose bisphosphate molecule is split into two molecules each containing 3 carbons as the backbone. FBP is split into two 3-carbon molecules called G3P, or glyceraldehyde 3-phosphate. Notice that the phos...
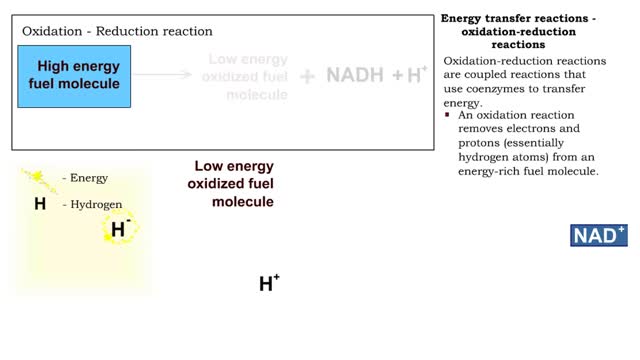
Types of energy transfer reactions: oxidation-reduction reactions and ATP generation reactions
By: HWC, Views: 12354
■ Metabolism balances anabolic and catabolic reactions. ■ Anabolism is energy transfer from ATP to simpler molecules in order to build them up into larger, more complex molecules. ■ Catabolism is breaking down larger, more complex molecules, usually to transfer energy from them in order...
Advertisement