Electromagnetic Spectrum, Chlorophyll and Pigment & Light
By: HWC
Date Uploaded: 04/01/2020
Tags: homeworkclinic.com Homework Clinic HWC Electromagnetic Spectrum Chlorophyll wavelengths radio waves X-rays ultraviolet light porphyrin ring
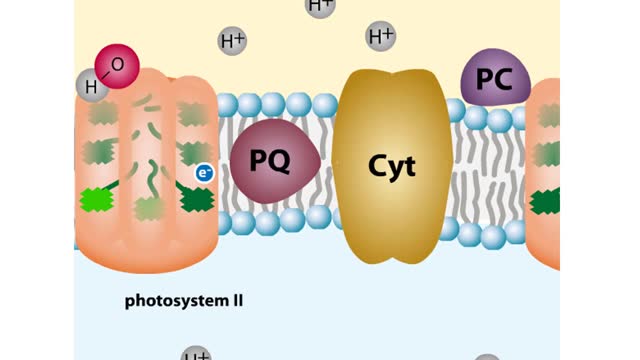
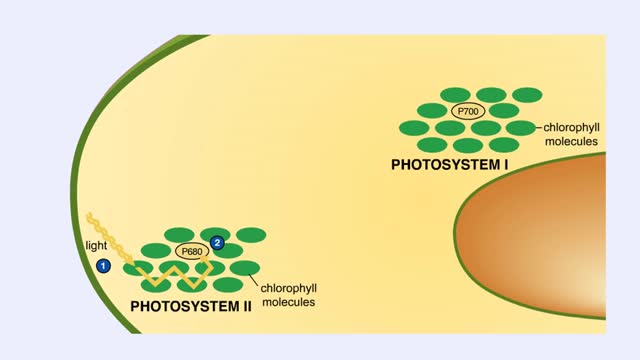
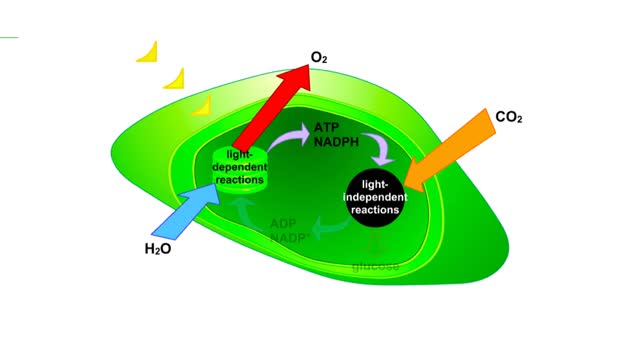
The sun gives off radiation that is called the electromagnetic spectrum. This is energy that travels as wavelengths and includes radio waves, X-rays and ultraviolet light. A portion of this radiation is known as visible light, and is the type of radiation that plants use to manufacture sugars. The smaller the wavelength, the greater the energy content. Pigments are organic molecules which reflect certain wavelengths of visible light. This is why leaves are "green" because the chlorophyll pigments in the leaves reflect green light. Flowers, fruits, and even animals have pigments which give them their individual colors. In addition to reflecting light, pigments also are able to absorb certain wavelengths. This makes them important molecules because this absorbing ability is actually a means of trapping energy from visible light. Plants, cyanobacteria and algae are able to convert this sunlight energy into the chemical bond energy of sugars. Let's look at the two types of chlorophyll molecules that are found in plants, chlorophyll a and chlorophyll b. The chlorophyll molecule consists of a head region consisting of four nitrogen-containing rings (called a porphyrin ring), and a hydrocarbon tail region. Chlorophylls are embedded in the internal thylakoid membranes of the chloroplast. The only difference between chlorophyll a and b is the nature of the "R" group: R is CHs for chlorophyll a and R is CHO for chlorophyll b. The graph that you see is the absorption curve for chlorophyll a. This means that chlorophyll a is absorbing visible light in those wavelengths where the curve is at its highest. Repeat the process for chlorophyll b.
Add To
You must login to add videos to your playlists.
Advertisement





Comments
0 Comments total
Sign In to post comments.
No comments have been posted for this video yet.