Major Elements in Biological Molecules: Lipids
By: HWC
Date Uploaded: 05/06/2020
Tags: homeworkclinic.com Homework Clinic HWC Lipids triglyceride triacylglycerol covalent ester bond fatty acid molecules hydrocarbon chain hydrophobic saturated fatty acid Palmitic acid oleic acid monounsaturated fatty acid Polyunsaturated fatty acids linoleic acid Mediterranean diet glycerol molecule tryglyceride ester covalent bonds Triglycerides monounsaturated polyunsaturated fatty acids
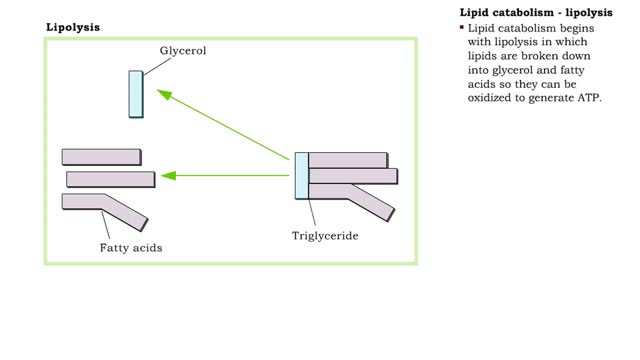
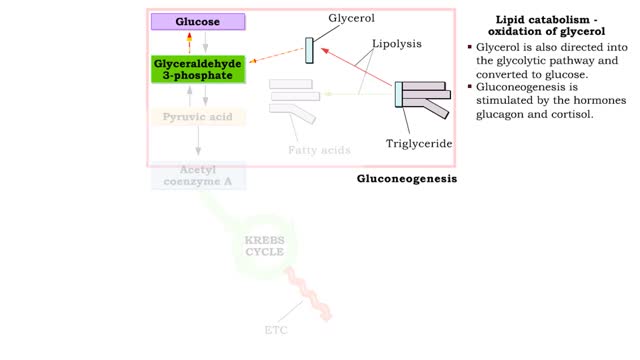
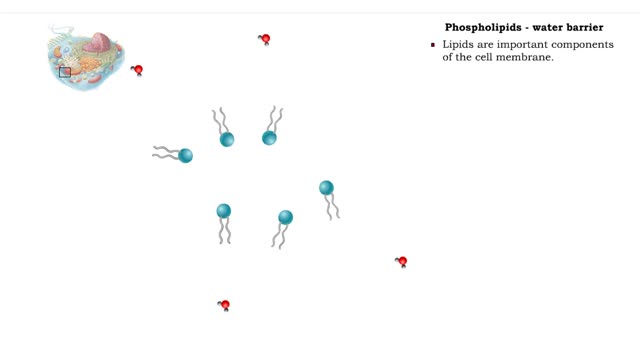
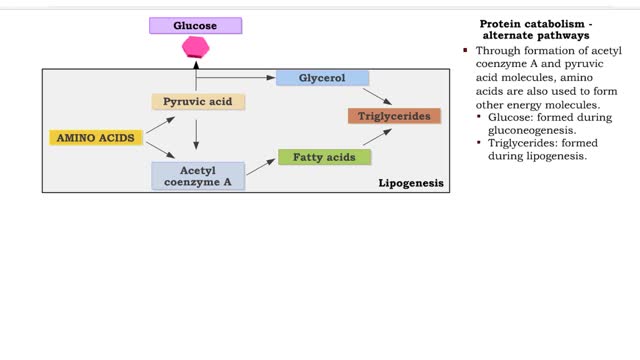
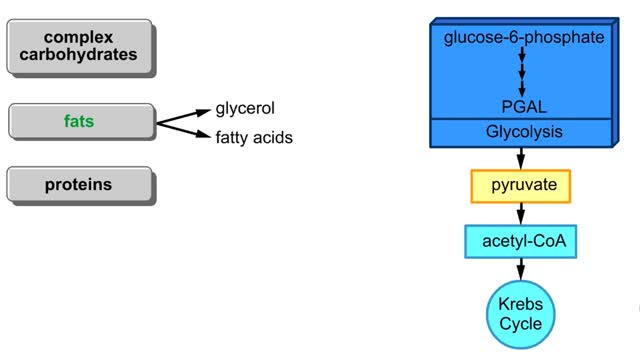
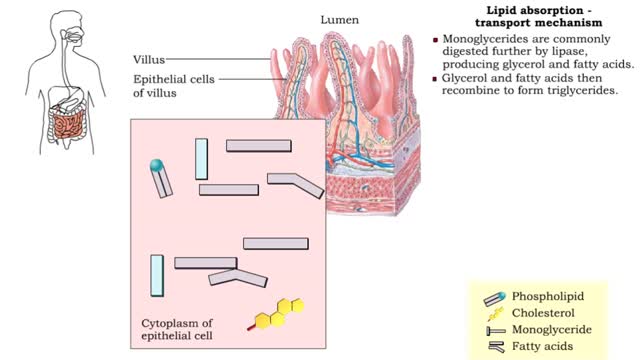
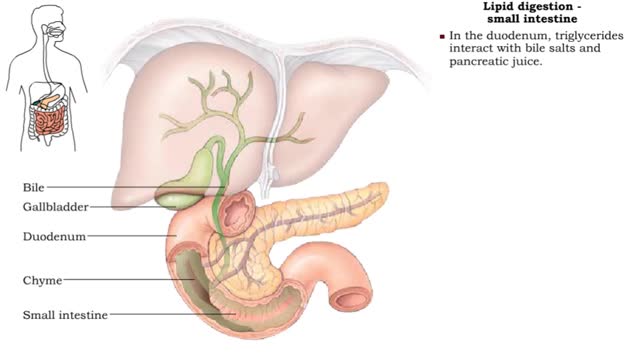
A triglyceride (also called triacylglycerol) is composed of three fatty acid molecules and one glycerol molecule. The fatty acids attach to the glycerol molecule by a covalent ester bond. The long hydrocarbon chain of each fatty acid makes the triglyceride molecule nonpolar and hydrophobic. Palmitic acid is a fatty acid with 16 carbon atoms. It is called a saturated fatty acid, because all the carbon atoms in the chain are single bonded to each other and are fully "saturated" with hydrogen atoms. Fatty acids, such as palmitic acid, are built by adding two carbon atoms at a time to smaller fatty acid molecules. A cell can use palmitic acid to form other fatty acids, such as oleic acid. In this process, oleic acid is formed by adding two carbon atoms to palmitic acid, and then by inserting a double bond between carbons 9 and 10. Because oleic acid has one double bond, it is considered a monounsaturated fatty acid. Polyunsaturated fatty acids, such as linoleic acid, have two or more double bonds. The double bonds kink these molecules and prevent them from packing tightly together. The loose packing results in triglycerides that are liquid at room temperature. Mammals cannot make linoleic acid; it is required in the diet. Oleic acid is the major component of olive oil. The Mediterranean diet, considered one of the healthiest, uses this oil as its primary source of lipid. A glycerol molecule makes up the backbone of a tryglyceride, in which glycerol's three hydroxyl (-OH) groups form ester covalent bonds with three fatty acid molecules. The covalent bonds form by condensation reactions in which water is a byproduct. Triglycerides can contain a mix of saturated, monounsaturated, and polyunsaturated fatty acids. They provide a concentrated store of energy.
Add To
You must login to add videos to your playlists.
Advertisement



Comments
0 Comments total
Sign In to post comments.
No comments have been posted for this video yet.