Activation Energy - Valence Electrons
By: HWC
Date Uploaded: 05/20/2020
Tags: homeworkclinic.com Homework Clinic HWC Activation energy kinetic energy valance electrons valence electrons
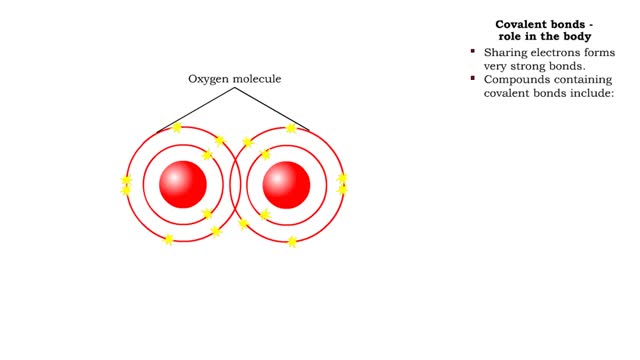
■ Shared electrons in the outermost orbital form bonds. These electrons are called valence electrons. ■ Valence electrons are disrupted and can be rearranged into a new bond. ■ The energy necessary to start a reaction and break bonds is called the activation energy. ■ Reactants have kinetic energy. ■ The reactants absorb more energy and activation energy is supplied. ■ As the products form, energy is released.
Add To
You must login to add videos to your playlists.
Advertisement



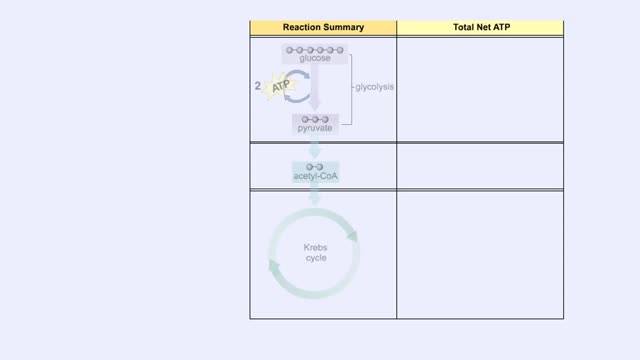
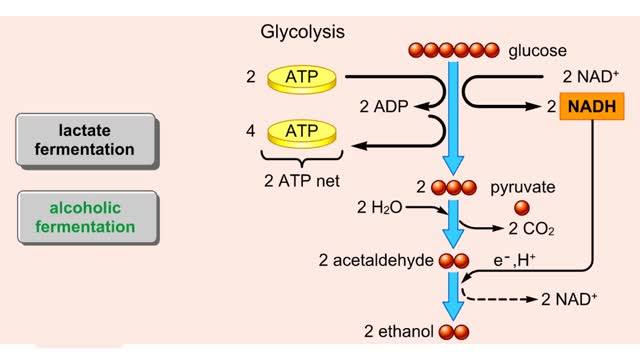
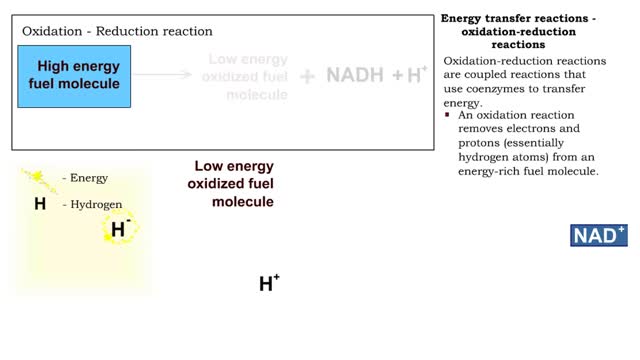
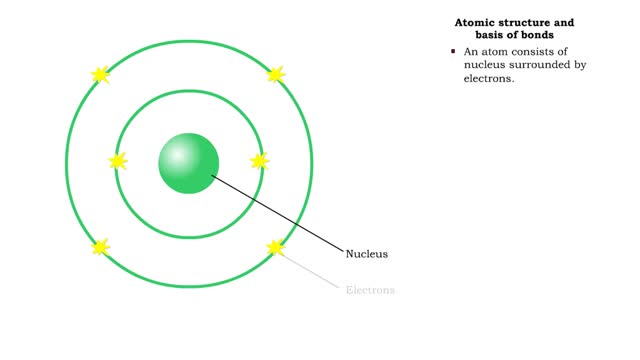
Comments
0 Comments total
Sign In to post comments.
No comments have been posted for this video yet.