Search Results
Results for: 'Factors that affect hydrogen bonds'
Hydrogen bonds - role in the body
By: HWC, Views: 11634
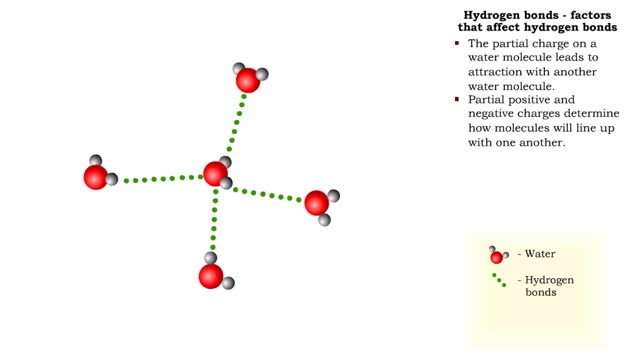
A hydrogen bond is the electromagnetic attraction between polar molecules in which hydrogen is bound to a larger atom, such as oxygen or nitrogen. This is not a sharing of electrons, as in a covalent bond. Instead, this is an attraction between the positive and negative poles of charged atoms. ...
By: HWC, Views: 10115
The slight positive charge of a hydrogen atom in a water molecule can attract an atom with a slight negative charge, such as the nitrogen in a molecule of ammonia. This forms a hydrogen bond between the two atoms. Hydrogen bonds join the two strands of a DNA molecule. Although hydrogen bo...
Bond in biological molecules (Ionic, Covalent and Hydrogen bonds)/ How atoms bond?
By: HWC, Views: 8603
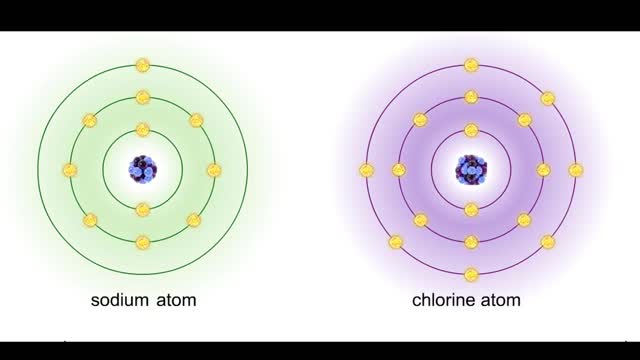
Sodium atoms and chloride atoms have unfilled orbitals in their outer shells. The lone electron in the outermost shell of a sodium atom can be pulled or knocked out. This ionizes the atom. It is now a positively charged sodium ion. A chlorine atom has an electron vacancy in its outer shell and...
By: HWC, Views: 5231
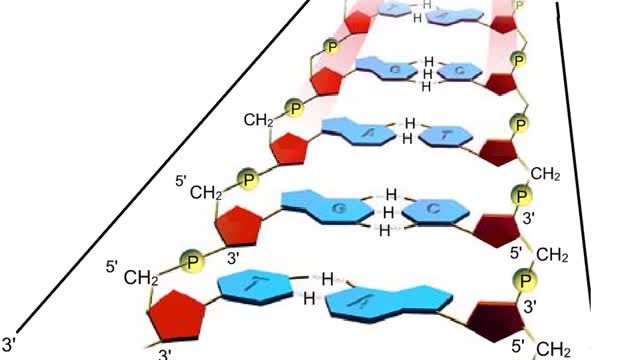
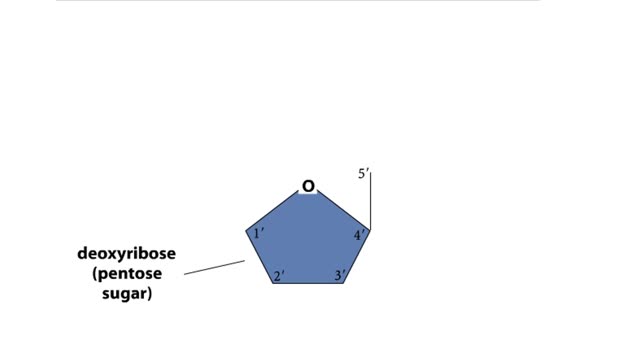
A section from a DNA double helix The backbone of each DNA strand consists of alternating deoxyribose sugars and phosphate groups. The two strands run in opposite directions. One runs from the 5' to 3' direction, the other in the 3' to 5' direction. Think of the deoxyribose units o...
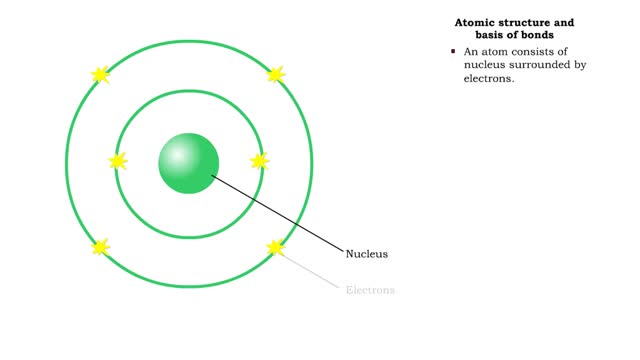
Bond types - Atomic structure and basis of bonds
By: HWC, Views: 11739
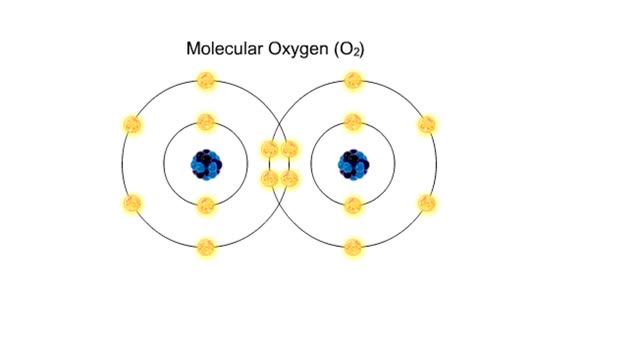
• Chemical bonds are fundamental to the structure and function of many types of molecules, such as proteins, carbohydrates, lipids, nucleic acids, gases, salts and water. ■ These molecules are composed of atoms that are held together by three different types of bonds. • The three types ...
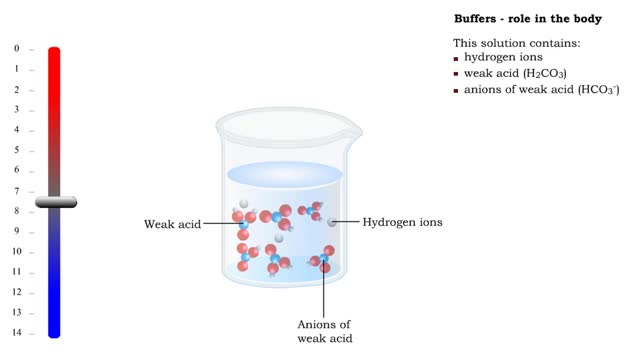
Buffers definition and the role of buffer in the body
By: HWC, Views: 11357
■ Too many H+ break hydrogen bonds and a protein comes apart. ■ Buffers react with excess H+ to protect proteins from breaking down. ■ Buffers consist of weak acid plus anions of that weak acid. This solution contains: • hydrogen ions • weak acid (H2CO3) • anions of we...
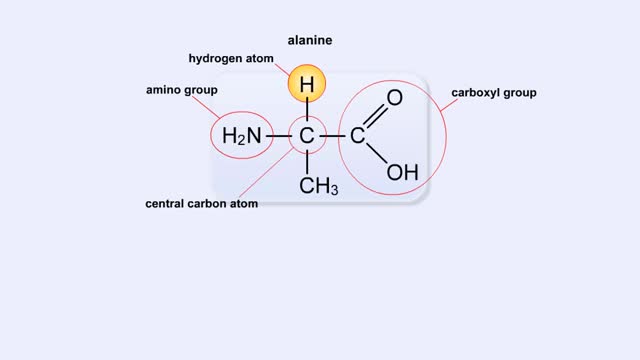
Structure of Amino Acid, Peptide Bonds & Polypeptides
By: HWC, Views: 10922
Here are the molecular formulas of three different amino acids. All amino acids share this backbone. The main difference between every amino acid is the side groups seen here, and these side groups give each of the amino acids their different characteristics. But before we get into that, let's ...
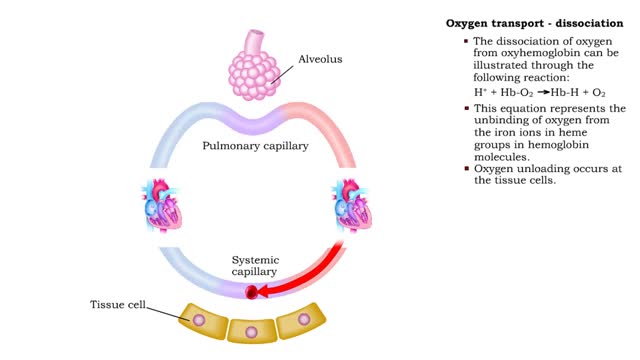
Oxygen transport: association and dissociation & Factors that affect hemoglobin's saturation with O2
By: HWC, Views: 11225
• The production of oxyhemoglobin can be illustrated through the following reaction: 02 + Hb-H --) Hb-02 + H+ • This equation represents the binding of oxygen to the iron ions in heme groups in hemoglobin molecules. • Oxygen binding or loading occurs at the lungs • The dissociatio...
Major Elements in Biological Molecules: Nucleic acids
By: HWC, Views: 11255
DNA and RNA are nucleic acids (polymers of nucleotides). Two polymers with complementary nucleotide sequences can pair with each other. This pairing endows nucleic acids with the ability to store, transmit, and retrieve genetic information. Two strands of DNA pair by hydrogen bonding. A compon...
Advertisement