Search Results
Results for: 'Properties of water'
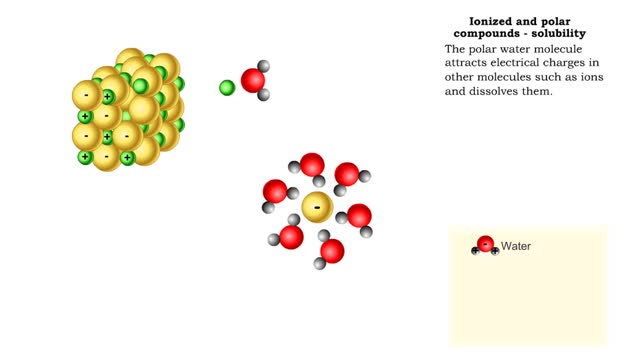
Properties of water -structure of water and polarity (Ionized and polar compounds)
By: HWC, Views: 11499
■ Water transports most of the molecules in the body. ■ The structure of a water molecule allows it to dissolve other molecules. ■ Shared electrons spend more time near the oxygen atom. ■ Oxygen end has a partial negative charge. ■ Hydrogen ends have a partial positive charge....
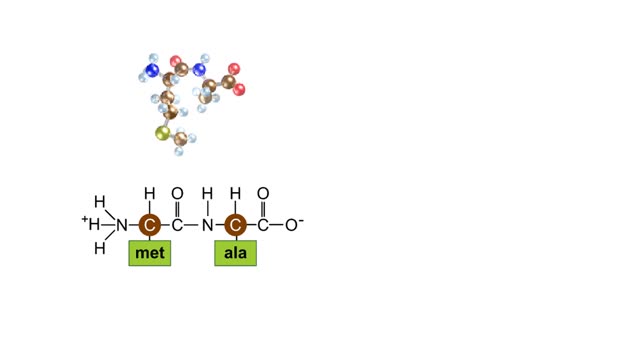
Peptide Bond Formation Animation
By: HWC, Views: 5145
During protein synthesis, peptide bonds link amino acids together in the order specified by DNA instructions. In this case, the first two amino acids in the protein are methionine and alanine. Here are ball-and-stick models of these amino acids. Peptide bond formation is a type of condensatio...
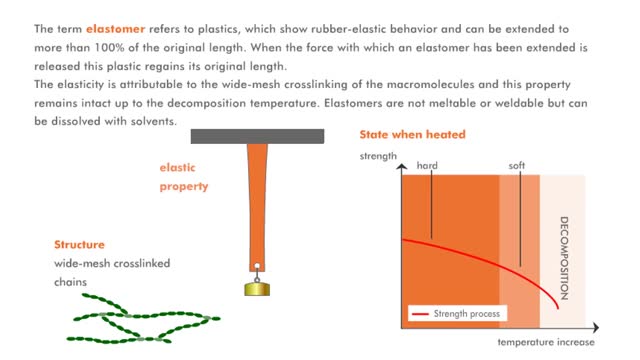
Properties of macromolecules (Explained with No Audio)
By: HWC, Views: 10265
The term thermoplast refers to a plastic, which when heated is soft and deformable, but which rehardens when cooled. The molecular structure of the macromolecules is comparable to a cotton ball in which the individual fibers of the macromolecules are shown. The fibers of the cotton ball can s...
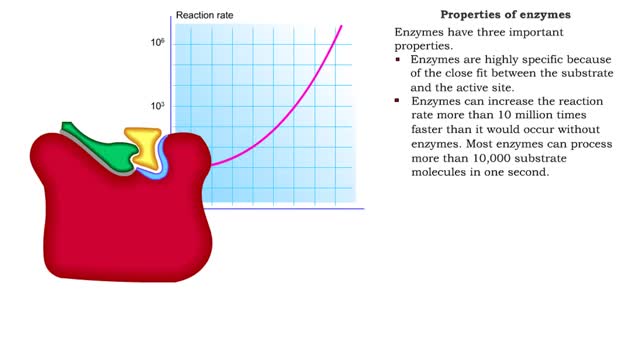
Enzyme structure - Properties of enzymes
By: HWC, Views: 11298
■ Enzymes are proteins that catalyze reactions. ■ Some enzymes have two parts: a protein or apoenzyme and a non-protein or cofactor. ■ Cofactor can be a metal ion or another organic molecule called a coenzyme. ■ Coenzymes often come from vitamins. ■ Cofactors affect the shape of...
By: HWC, Views: 11492
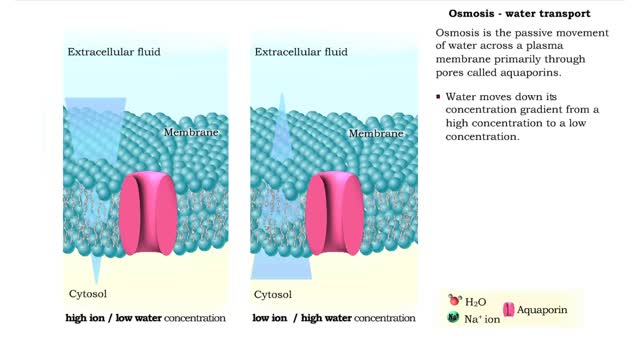
Osmosis is the flow of water down its concentration gradient, across a semi-permeable membrane. Osmosis is an example of diffusion, which is when molecules tend to distribute themselves evenly in a space. what is a semi-permeable membrane? It is a membrane or barrier that allows some molec...
By: HWC, Views: 10685
Observe the burning logs of wood. The logs burn to emit heat, light and carbon dioxide. What is left behind is ash. This residue is a new substance with a different molecular structure than the original wood. Similarly when baking the dough into bread, it becomes fluffy and light. There is a ch...
Non-polar compounds - insolubility
By: HWC, Views: 11432
• A non-polar molecule has uniform distribution of electrons. • Non-polar compounds like fatty acids in lipids have a high proportion of carbon and hydrogen. • Lipids possess no charge or partial charge. • Lipids are not attracted to water molecules. • Lipids are not soluble in...
Antidiuretic hormone (vasoconstriction, water reabsorption & sweat inhibition)
By: HWC, Views: 11187
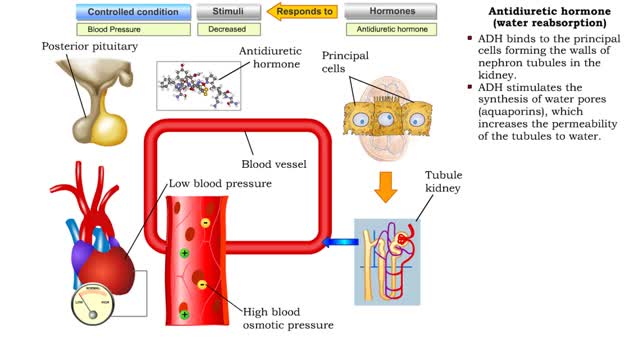
• Dehydration, blood loss, and low amounts of water in the blood can cause blood volume and pressure to decrease. • Neurosecretoxy cells in the posterior pituitary release antidiuretic hormone(ADH). • ADH binds to smooth muscle cells in blood vessel walls, stimulating them to vasoconstr...
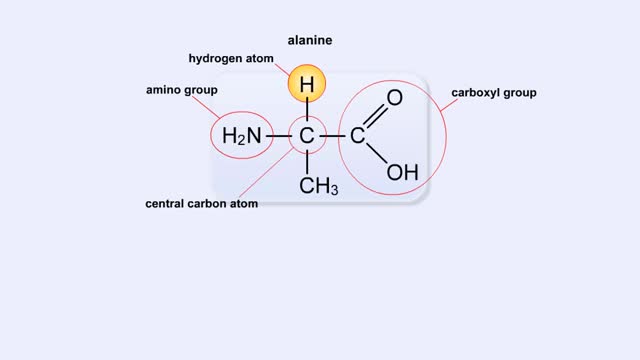
Structure of Amino Acid, Peptide Bonds & Polypeptides
By: HWC, Views: 10931
Here are the molecular formulas of three different amino acids. All amino acids share this backbone. The main difference between every amino acid is the side groups seen here, and these side groups give each of the amino acids their different characteristics. But before we get into that, let's ...
Advertisement