Search Results
Results for: 'enzyme carbonic anhydrase'
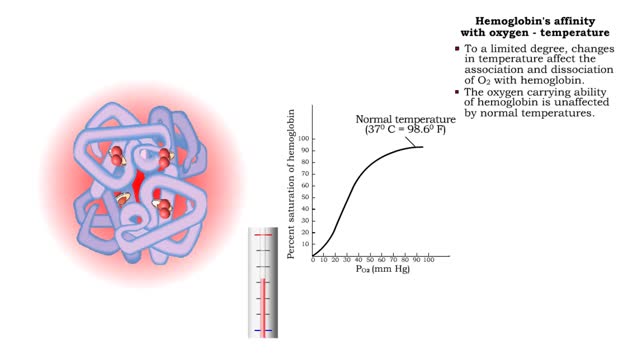
Hemoglobin's affinity with oxygen - carbon dioxide, temperature and bisphosphoglycerate (BPG)
By: HWC, Views: 11297
• The carbon dioxide gas is temporarily converted to carbonic acid in red blood cells by the enzyme carbonic anhydrase, and then further converted to hydrogen and bicarbonate ions. • The result of increased carbon dioxide is decreased pH causing the Bohr effect. • Elevated carbon dioxid...
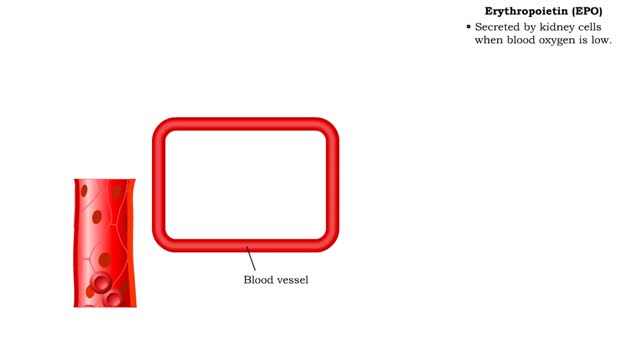
Red Blood Cells - Erythropoietin (EPO)
By: HWC, Views: 11236
• The endocrine system maintains many body conditions within normal limits with feedback loops. Each endocrine feedback loop maintains homeostasis using the following components: • Stimulus - a change in a body condition. • Production cell - an endocrine cell that produces a hormone aft...
By: HWC, Views: 10782
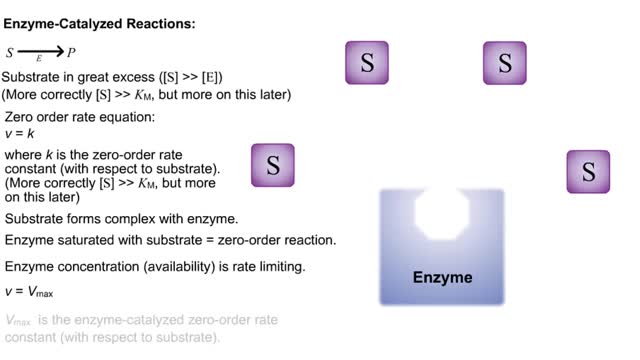
S P Substrate in great excess ([S] -- [E]) (More correctly [S] -- KM, but more on this later) Zero order rate equation: v = k where k is the zero-order rate constant (with respect to substrate). (More correctly [S] -- KM, but more on this later) Substrate forms complex with enzyme. ...
Virtual Enzyme Kinetics & Lineweaver Burk Plot
By: HWC, Views: 10878
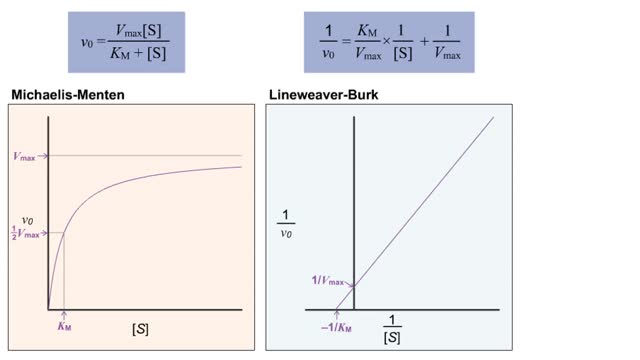
• The double-reciprocal (also known as the Lineweaver-Burk) plot is created by plotting the inverse initial velocity (1/V0) as a function of the inverse of the substrate concentration (1/[S]). • This plot is a useful way to determined different inhibitors such as competitive, uncompetitive...
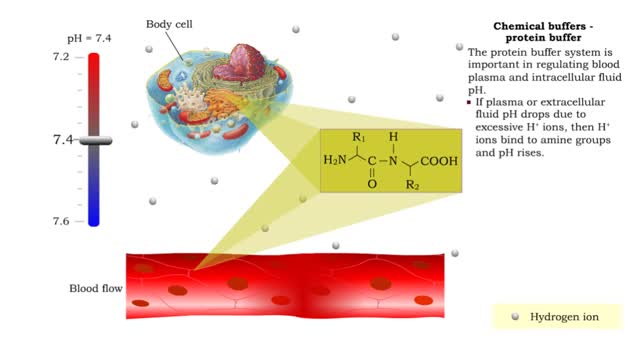
Chemical Buffers - protein buffer, phosphate buffer system and bicarbonate buffer system
By: HWC, Views: 11515
• There are a variety of chemicals in body fluids that prevent the fluids from undergoing large changes in. • These chemicals buffer or regulate fluctuations in H+ concentration. • Chemical buffers: • Bind to H+ ions when there are too many in a solution so pH remains normal. •...
By: HWC, Views: 11160
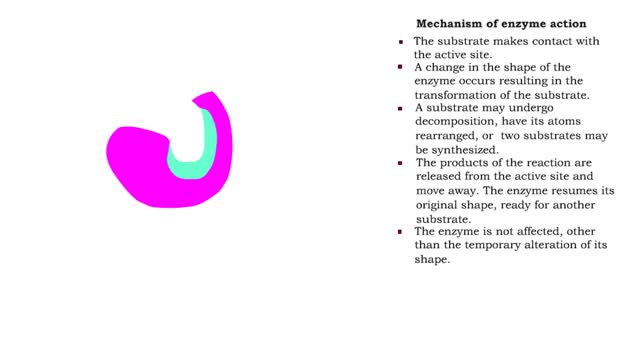
■ The substrate makes contact with the active site. ■ A change in the shape of the enzyme occurs resulting in the transformation of the substrate. ■ A substrate may undergo decomposition, have its atoms rearranged, or two substrates may be synthesized. ■ The products of the reaction...
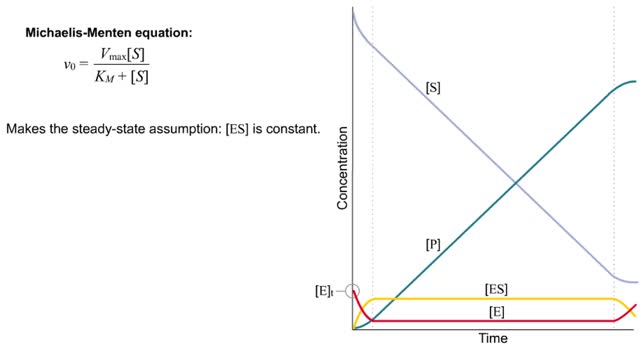
Michaelis–Menten equation & Kinetic parameters
By: HWC, Views: 11159
The Michaelis–Menten equation is the rate equation for a one-substrate enzyme-catalyzed reaction. This equation relates the initial reaction rate (v0), the maximum reaction rate (Vmax), and the initial substrate concentration [S] through the Michaelis constant KM—a measure of the substrat...
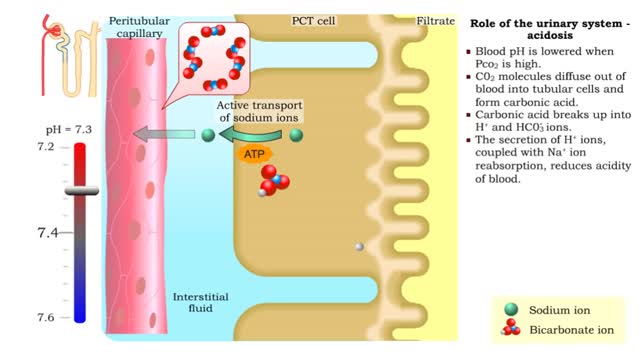
Role of the urinary system - acidosis and alkalosis
By: HWC, Views: 11488
• Tubular cells of the proximal convoluted tubule and collecting tubules can alter filtrate pH and therefore blood pH. • These cells can affect blood pH with two coupled mechanisms: • Reabsorption of bicarbonate ions. • Secretion of hydrogen ions. • The reabsorption of bicarbonate...
By: HWC, Views: 10503
✔ https://HomeworkClinic.com ✔ https://Videos.HomeworkClinic.com ✔ Ask questions here: https://HomeworkClinic.com/Ask Follow us: ▶ Facebook: https://www.facebook.com/HomeworkClinic ▶ Review Us: https://trustpilot.com/review/homeworkclinic.com Kinetics is the study of the rat...
Advertisement