Hemoglobin's affinity with oxygen - carbon dioxide, temperature and bisphosphoglycerate (BPG)
By: HWC
Date Uploaded: 10/31/2019
Tags: homeworkclinic.com Homework Clinic HWC Hemoglobin's affinity with oxygen enzyme carbonic anhydrase Bohr effect oxyhemoglobin metabolizing cells alveolar spaces hemoglobin blood temperature aerobic metabolism bisphosphoglycerate (BPG) Red blood cells mitochondria glycolysis thyroxine epinephrine norepinephrine testosterone
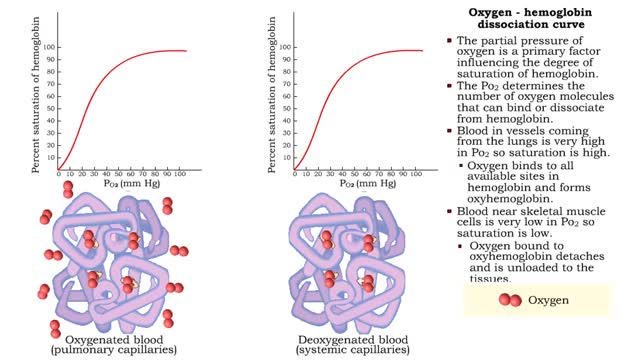
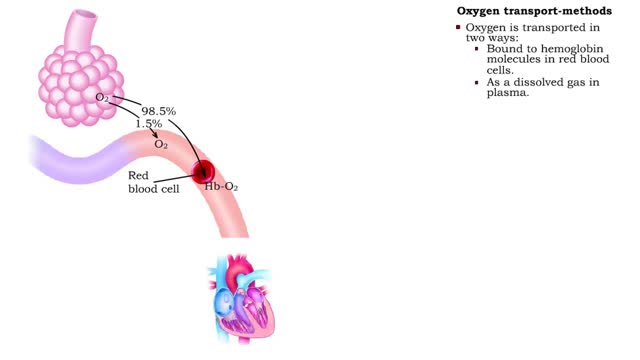
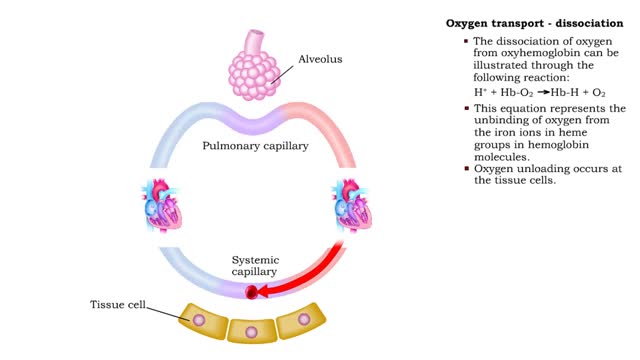
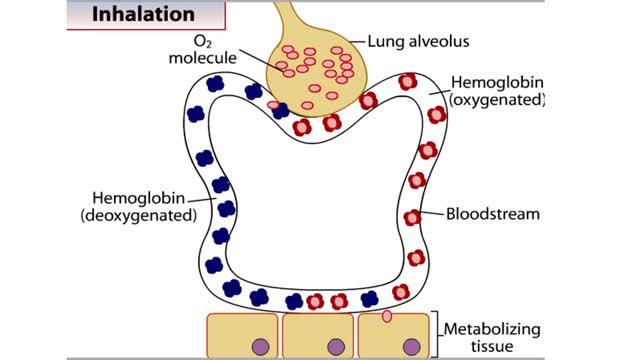
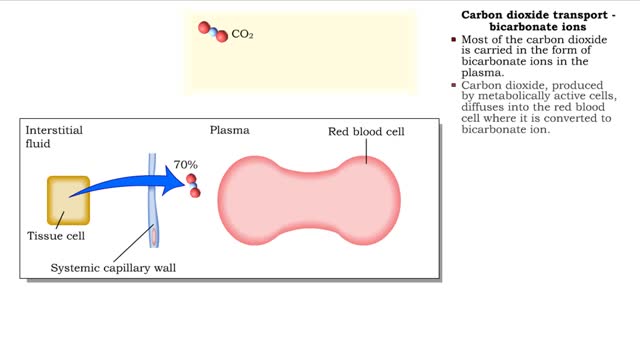
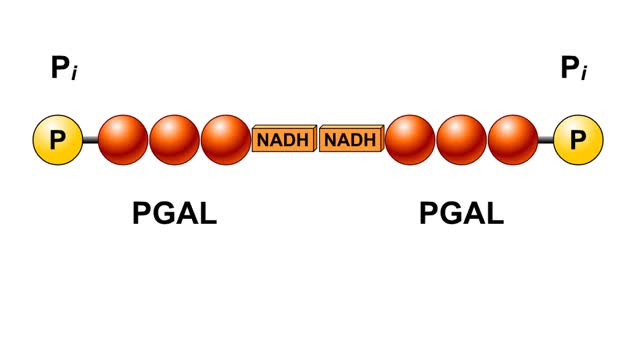
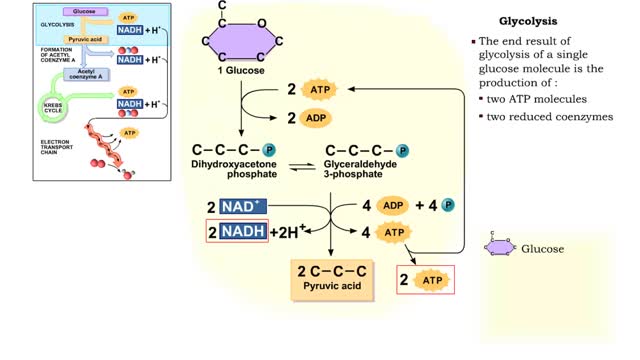
• The carbon dioxide gas is temporarily converted to carbonic acid in red blood cells by the enzyme carbonic anhydrase, and then further converted to hydrogen and bicarbonate ions. • The result of increased carbon dioxide is decreased pH causing the Bohr effect. • Elevated carbon dioxide levels enhance unbinding of oxygen from oxyhemoglobin thereby making oxygen available for actively metabolizing cells. • By contrast, decreased carbon dioxide, as in the alveolar spaces, increases affinity of hemoglobin for oxygen and promotes oxygen loading and transport. • To a limited degree, changes in temperature affect the association and dissociation of O2 with hemoglobin. • The oxygen carrying ability of hemoglobin is unaffected by normal temperatures. • Near metabolically active cells, blood temperature rises, increasing the thermal motion of molecules which promotes the unloading of O2 to continue fueling aerobic metabolism in the tissue cells. • When temperature lowers, metabolism slows and the need for O2 in cells lessens. More O2 remains bound to the hemoglobin. • Red blood cells do not have mitochondria so they do not undergo aerobic metabolism, using only glycolysis to generate ATP. • A special product of glycolysis, bisphosphoglycerate, or BPG, accumulates in red blood cells in low oxygen situations. • The hormones thyroxine, human growth hormone, epinephrine, norepinephrine, and testosterone can increase the production of BPG. • The higher the level of BPG in the red blood cells, the more O2 that is unloaded from the hemoglobin.
Add To
You must login to add videos to your playlists.
Advertisement



Comments
0 Comments total
Sign In to post comments.
No comments have been posted for this video yet.